Unveiling the Mysteries: Conservation of Matter and Convertibility of States
The Immutable Law of Matter
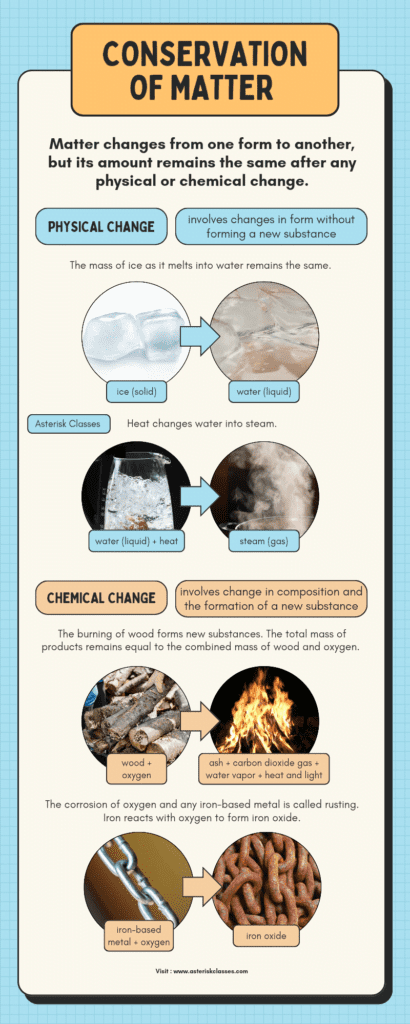
Matter, the substance that composes all things in our observable universe, is governed by a fundamental principle known as the Law of Conservation of Matter. This law asserts that matter is neither created nor destroyed; it simply changes form. Whether through physical changes like melting and freezing, or chemical changes such as combustion or oxidation, the total amount of matter remains constant within a closed system.
Physical Changes: A Matter of State
When matter undergoes a physical change, it transitions between different states—solid, liquid, and gas—without altering its core chemical structure. For instance, water can exist as ice, liquid water, or steam, but its molecular composition of two hydrogen atoms bonded to one oxygen atom remains unchanged through these transformations.
Chemical Changes: Bonds Broken and Formed
Chemical changes, on the other hand, involve the breaking and forming of atomic bonds, resulting in substances with entirely new chemical properties. Despite these profound transformations, the total mass of the reactants equals the mass of the products, adhering to the conservation principle.
Convertibility: The Dynamic States of Matter
The states of matter—solid, liquid, and gas—are not permanent fixtures. They are interconvertible, transitioning from one state to another through the application or removal of energy, typically in the form of heat.
Heating Up: Solids to Liquids to Gases
Heating a solid can turn it into a liquid, as seen when ice melts into water. Further heating converts the liquid into a gas, like boiling water into steam. This process is driven by the increase in molecular energy, allowing particles to move more freely.
Cooling Down: Gases to Liquids to Solids
Conversely, cooling a gas can condense it into a liquid, and further cooling can freeze it into a solid. This is evident when water vapor in the air condenses into droplets and further freezes into ice crystals.
The Implications for Science and Sustainability
Understanding the conservation of matter and the convertibility of states is crucial for scientific endeavors and environmental sustainability. It allows us to predict the outcomes of chemical reactions, design efficient materials, and manage resources responsibly.
In the Laboratory: Precision and Prediction
In chemical laboratories, the law of conservation of matter is paramount for predicting the amounts of substances produced in reactions, ensuring precise measurements and efficient use of materials.
In the Environment: Resource Management
Environmental conservation efforts benefit from this knowledge by enabling the recycling of materials and minimizing waste. The immutable cycle of matter supports the concept of a circular economy, where nothing is truly lost but transformed and reused.
Conclusion: The Perpetual Dance of Matter
The conservation of matter and the convertibility of states are not just scientific concepts; they are the very rules that dictate the perpetual dance of matter in our universe. They remind us that everything around us is part of a larger, unending cycle of transformation—a cycle that sustains life, drives innovation, and preserves the balance of our world.
The exploration of matter’s conservation and its dynamic states reveals the intricate and awe-inspiring nature of the physical world. It underscores the importance of scientific laws in understanding the universe and highlights the potential for human ingenuity to work in harmony with these natural principles for a sustainable future.