 >
>Understanding the States of Matter: Properties and Features
Introduction
Matter is anything that has mass and occupies space. It can exist in several distinct states, each with unique properties and features. In this blog post, we’ll delve into the three primary states of matter—solids, liquids, and gases—and explore their characteristics with examples and facts.
Solid State
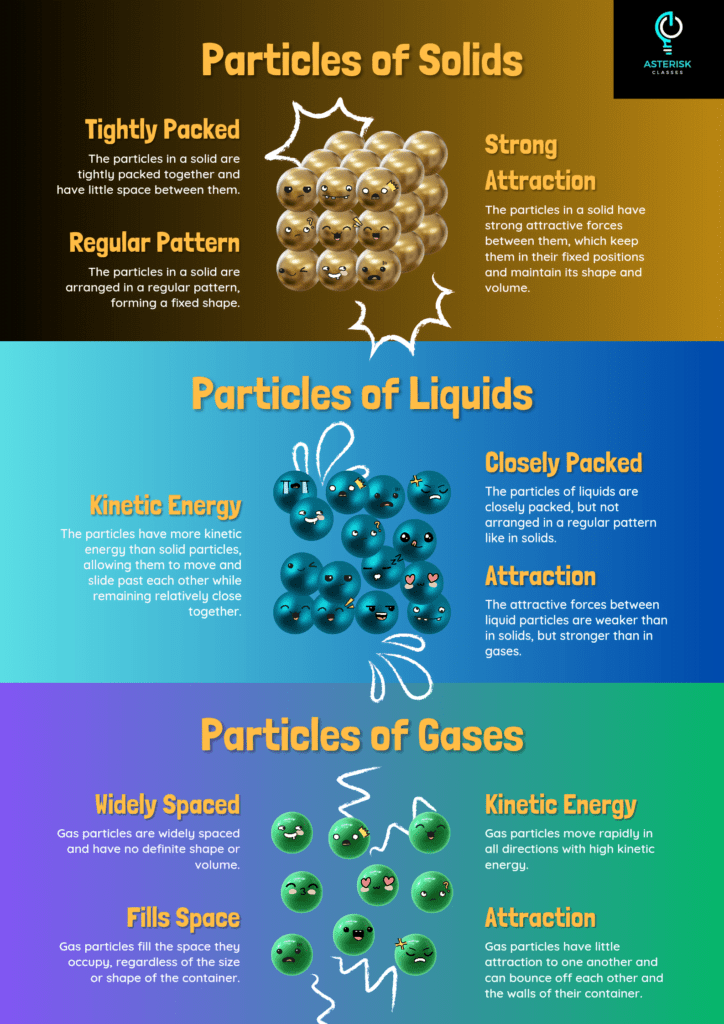
Properties and Features
Solids are defined by their fixed shape and volume. The particles in a solid are closely packed together and vibrate in place, which accounts for their rigidity. One of the key properties of solids is their structural stability, which makes them maintain their shape under standard conditions.
Examples and Facts
A classic example of a solid is a diamond, which is renowned for its hardness. This property arises from the strong covalent bonds between carbon atoms in a lattice structure. Another example is ice, the solid state of water, which forms when water molecules are cooled and arrange themselves into a crystalline structure.
Liquid State
Properties and Features
Liquids have a definite volume but no fixed shape. They take the shape of their container due to the particles being closely packed but not fixed in place. This allows liquids to flow and conform to the shape of their surroundings.
Examples and Facts
Water is the most common example of a liquid, exhibiting properties such as surface tension and viscosity. Mercury is another interesting liquid, notable for being a metal that remains in liquid form at room temperature due to weak bonding between its atoms.
Gaseous State
Properties and Features
Gases have neither a fixed shape nor a fixed volume. They expand to fill their container, and their particles are far apart and move freely. This high kinetic energy and low density make gases compressible.
Examples and Facts
The air we breathe is a mixture of gases, primarily nitrogen and oxygen. Helium is a lighter-than-air gas used in balloons, and its low density is due to the small size and mass of its atoms, allowing it to rise in the atmosphere.
Conclusion
The states of matter are a fundamental concept in physics and chemistry, each with its own set of properties and behaviors. Understanding these can provide insights into the nature of materials and their potential applications. From the rigidity of solids to the fluidity of liquids and the expansiveness of gases, the states of matter continue to fascinate and inspire scientific exploration.