The crystalline solids can be categorized into the following four categories, depending on the nature of the intermolecular forces functioning in them.
1. Molecular solids 2. Ionic solids 3. Metallic solids 4. Covalent or network solids
1. Molecular solids
- These crystalline crystals are made up of molecules. The molecules are held together by dispersion forces, London forces, dipole-dipole forces, or hydrogen bonds.
They are further classified into the following categories:
(a) Nonpolar molecular solids
These are crystalline solids composed of either noble gas atoms (helium, neon, argon, etc.) or non-polar molecules.
Examples include solid helium, solid argon, solid hydrogen, solid carbon dioxide, and iodine. In these materials, atoms or molecules are held together by modest dispersion forces known as London forces.
 >
>
These solids have the following characteristics:
(i) They are generally soft.
(ii) They have lower melting points.
(iii) They are typically liquid or gaseous at room temperature and pressure.
(iv) Because they contain neutral molecules in both solid and dissolved forms, they are non-conductors of electricity.
(b) Polar molecular solids
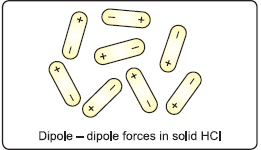
These are made up of molecules of different substances that have polar covalent bonds. Examples are solid HCl, solid SO2, solid NH3, and so on. The molecules in such materials are held together by significantly greater dipole-dipole interactions. They exhibit characteristics similar to those of non-polar covalent compounds, as explained below:
 >
>
(i) The solids are soft, too.
(ii) They are not electrical conductors.
(iii) They have lower melting and boiling points. However, the melting and boiling temperatures are higher than those of non-polar molecular solids.
(iv) Because their melting and boiling temperatures are not particularly high, the majority of them are gaseous or liquid at ambient temperature and pressure.
(c) Hydrogen-bound molecular solids
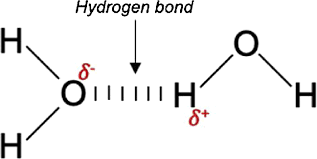
Such materials have hydrogen linkages between their molecules. In solid water (ice), the negative end of one molecule (Oδ-) attracts the positive end of a neighbouring molecule (Hδ+), forming a hydrogen bond between O and H. These solids have hydrogen bonds between H and highly electronegative elements like F, O, or N.
 >
>
These solids possess the following characteristics:
(i) At room temperature and normal pressure, they are often volatile liquids or soft solids.
(ii) They are not electrical conductors.
(iii) They frequently have higher melting and boiling temperatures than non-polar and polar molecular solids
2. Ionic Solids
Ionic solids are composed of positively and negatively charged ions that are distributed throughout the solid in a consistent manner. The ions are bound together by strong coulombic (or electrostatic) forces. Thus, in ionic solids, the component particles are ions.
 >
>
The uniform arrangement of ions continues throughout the crystal. For example, in sodium chloride, Na+ and Cl- ions have a definite regular configuration. In this configuration, each Na+ is surrounded by six Cl- ions, and each Cl- is surrounded by six Na+ ions. Similar regular groupings are also found in other ionic solids.
The main characteristics of ionic crystals are:
(i) Ionic solids are exceedingly rigid and brittle.
(ii) They have exceptionally high melting and boiling points.
(iii) They are poor conductors of electricity and, thus, are insulators in solid state. This is because solid ions cannot move freely. However, in the molten state or when dissolved in water (aqueous solution), the ions become free to roam around and conduct electricity.
(iv) They have high enthalpies of vaporization.
(v) Ionic crystals are soluble in water, as well as in other polar solvents.
They are insoluble or very marginally soluble in non-polar solvents such as benzene, carbon tetrachloride, and carbon disulfide.
The main examples of ionic crystals are salts like NaCl, KNO3, LiF, Na2SO4, etc.
3. Metallic solids or crystals
In metallic crystals, the component particles are positive ions (called kernels) immersed in a sea of mobile electrons. The electrons in Metallic crystals are mobile and uniformly dispersed throughout the crystal.
Each metal atom contributes one or more electrons to this sea of mobile electrons. These free mobile electrons are responsible for the high electrical and thermal conductivities of metals. When an electric field is provided, these electrons flow through the network of positive ions (called kernels). Similarly, when heat is delivered to one area of a metal, the thermal energy is uniformly spread throughout the crystal by free electrons.
The forces existing between the metal ions are called metallic bonds. The major properties of metallic crystals are: (i) Metallic crystals may be firm as well as soft.
(ii) They are excellent conductors of heat and electricity.
(iii) They have a metallic shine and color in rare circumstances.
(iv) They are malleable and ductile. Due to their pliable nature, they can be hammered into sheets and dragged into wires.
(v) They have modest enthalpies of fusion.
Common metals such as nickel, copper, and alloys are examples of metallic crystals.
4. Covalent or network solids or crystals
In covalent crystals, the component particles are non-metal atoms, which are linked to the neighboring atoms by covalent bonds throughout the crystal. In other words, there is a continuous network of covalent bonds generating a gigantic three-dimensional structure. They are also referred to as giant molecules.
Covalent bonds are strong and directed in nature, and so the atoms in these substances are held extremely tightly at their places.
The key characteristics of covalent crystals are: (i) The covalent crystals are hard.
(ii) They have exceptionally high melting points and may even decompose before melting.
(iii) They are poor conductors of electricity and are insulators.
The main examples of covalent crystals include diamond, carborundum (silicon carbide), quartz (SiO2), boron nitride (BN), etc.
Diamond
A diamond is a classic example of a covalent crystal. It has a network structure consisting of a relatively high number of carbon atoms connected to each other. Each carbon atom is coupled to four other carbon atoms by single covalent bonds., so that each carbon atom resides at the core of a regular tetrahedron and the other four carbon atoms are located at the corners of the tetrahedron.
Therefore, there is a three-dimensional network with strong covalent bonds. As a result, diamond is a tough crystal with a very high melting point (3843 K). Since all the valence electrons of carbon are tightly trapped in carbon-carbon bonds, diamond is a poor conductor of electricity.
 >
>Graphite
Graphite is also a covalent solid, but it is soft and a strong conductor of electricity. In graphite, carbon atoms are grouped into distinct layers consisting of other nearby carbon atoms by single covalent connections in the same layer.
Each carbon atom’s fourth valence electron is present between different layers and is free to roam about. Because graphite has free electrons, it is an excellent electrical conductor. Further, the distinct layers are separated by a wide distance (340 pm), which is higher than the carbon-carbon bond length. This means that only weak van der Waals forces are present between these layers, and hence, these layers can glide one over the other.
This makes graphite a soft and solid lubricant.