Allotropes of Carbon
Structures, Properties, and Applications
Introduction
Carbon, with its atomic number 6 and electronic configuration of 2,4, exhibits a remarkable property known as allotropy. Allotropes are different structural forms of the same element in the same physical state. This phenomenon arises due to carbon’s ability to form various types of bonds and structures, leading to distinct physical and chemical properties among its allotropes.
Carbon’s allotropy stems from its tetravalency and unique ability to form stable covalent bonds with itself and other elements. This blog post explores the major allotropes of carbon, their structures, properties, and applications, with a focus on preparing students for examinations.
Exam Tip:
Questions about carbon allotropes frequently appear in chemistry examinations. Be prepared to:
- Compare and contrast different allotropes
- Explain their structural differences
- Describe how structure influences properties
- Discuss their applications in various fields
Key Allotropes of Carbon
1. Diamond

Diamond’s tetrahedral crystal structure
Structure:
- Each carbon atom is covalently bonded to four other carbon atoms
- Forms a three-dimensional tetrahedral structure
- Bond angle: 109.5° (sp³ hybridization)
- Crystallizes in a face-centered cubic lattice
Physical Properties:
- Hardest naturally occurring substance (10 on Mohs scale)
- Very high melting point (~3550°C) and boiling point
- Excellent thermal conductor
- Electrical insulator (no free electrons)
- High refractive index (brilliance)
- Density: 3.5 g/cm³
Applications:
- Jewelry and gemstones
- Industrial cutting and drilling tools
- Heat sinks in electronics
- Abrasives for polishing
- High-pressure scientific applications
Exam Focus:
Understand why diamond is hard (strong covalent bonds in all directions) yet brittle (bonds can break when enough force is applied along specific planes).
2. Graphite
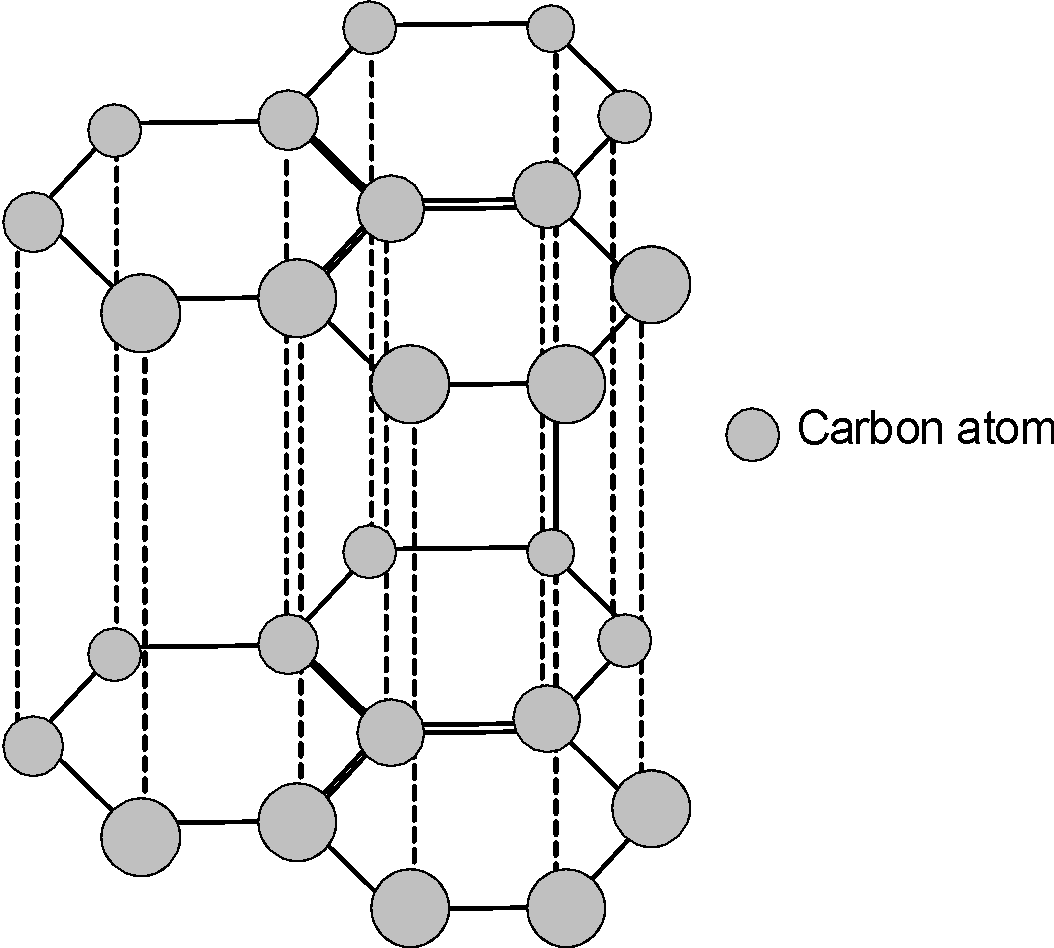
Graphite’s layered hexagonal structure
Structure:
- Each carbon atom is covalently bonded to three other carbon atoms
- Forms flat, hexagonal layers (sheets)
- Bond angle: 120° (sp² hybridization)
- Layers held together by weak van der Waals forces
- Delocalized π-electrons above and below each sheet
Physical Properties:
- Soft and slippery (layers can slide over each other)
- High melting point (~3650°C)
- Good electrical conductor (due to delocalized electrons)
- Good thermal conductor within layers, poor between layers
- Opaque with metallic luster
- Density: 2.2 g/cm³
Applications:
- Pencil lead (leaves marks when rubbed on paper)
- Lubricants (due to its slippery nature)
- Electrodes in batteries and electrolysis
- Crucibles for high-temperature metallurgical processes
- Moderator in nuclear reactors
Exam Focus:
Understand that graphite’s electrical conductivity is due to the fourth valence electron of each carbon atom being delocalized and free to move within the sheet structure.
3. Fullerenes
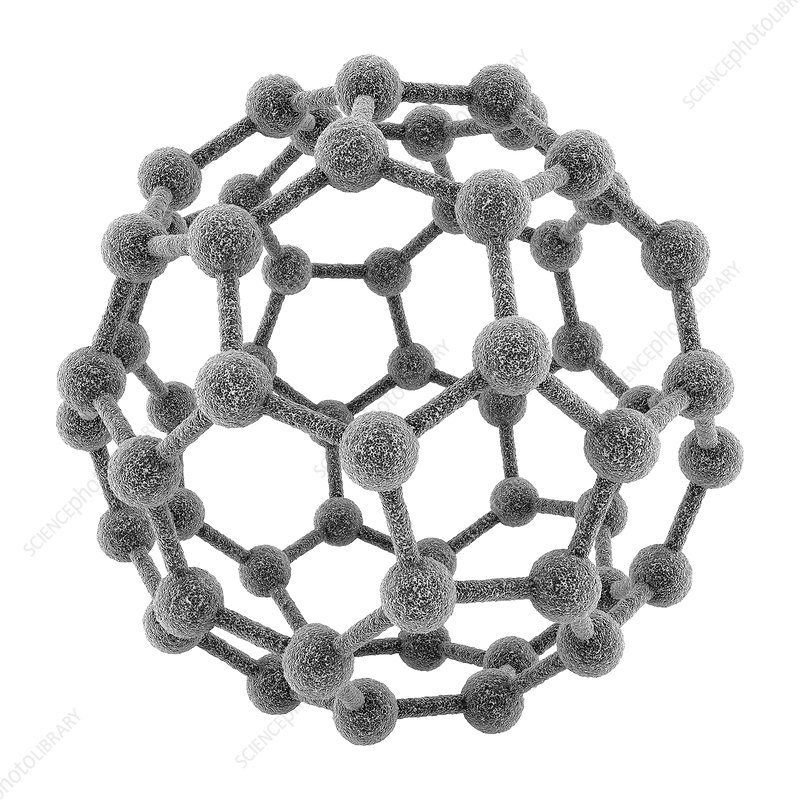
Buckminsterfullerene (C₆₀) structure
Structure:
- Hollow, cage-like molecules with carbon atoms arranged in pentagonal and hexagonal rings
- Most common is Buckminsterfullerene (C₆₀) with 60 carbon atoms
- Carbon atoms are sp² hybridized
- Resembles a soccer ball with 20 hexagons and 12 pentagons
- Other forms include C₇₀, C₇₆, C₈₄, etc.
Physical Properties:
- Soluble in organic solvents
- Stable molecular structure
- Semiconductor behavior
- Low reactivity (but can undergo addition reactions)
- Dark solid at room temperature
Applications:
- Superconductors (when doped with alkali metals)
- Drug delivery systems in medicine
- Lubricants
- Solar cell technology
- Potential antioxidants and anti-HIV agents
Exam Focus:
Remember that fullerenes were discovered in 1985 by Robert Curl, Harold Kroto, and Richard Smalley, who received the 1996 Nobel Prize in Chemistry for this discovery.
4. Graphene
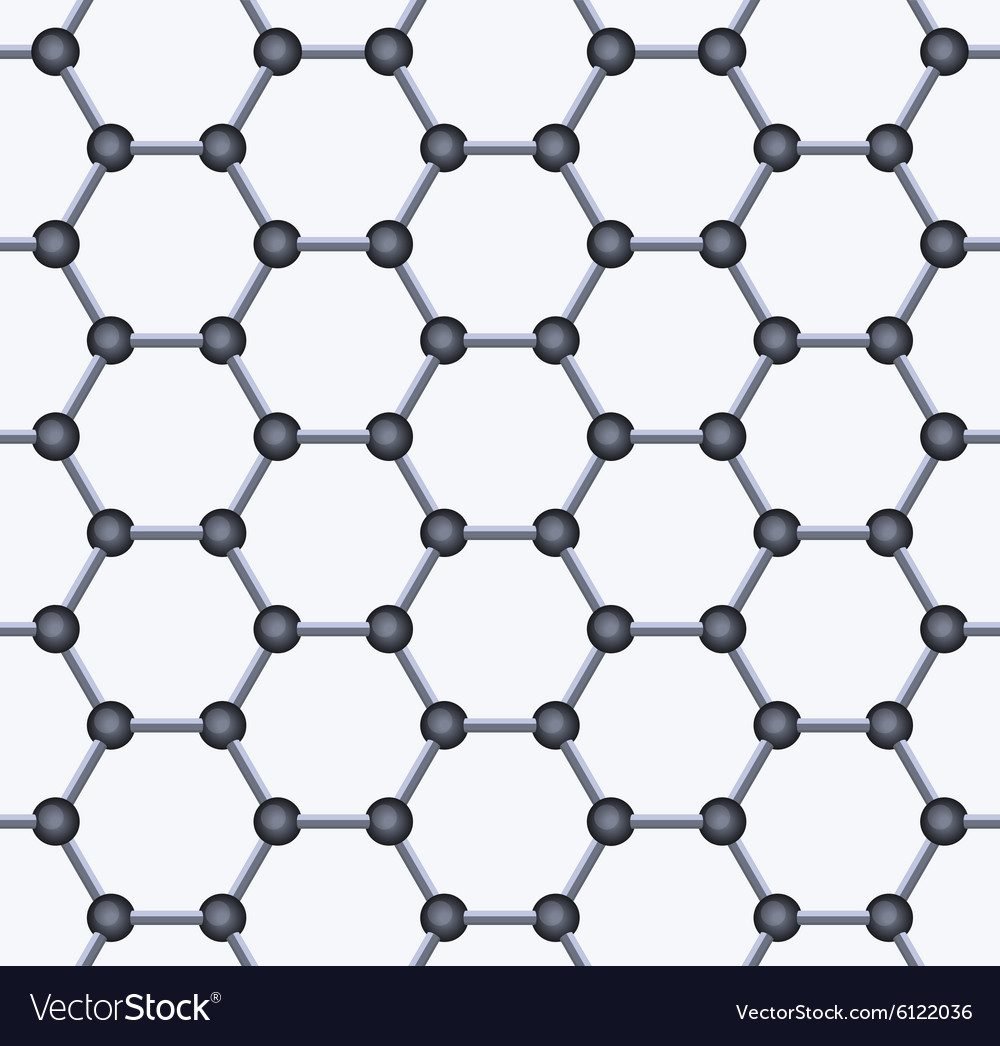
Single-layer graphene structure
Structure:
- Single layer of carbon atoms arranged in a hexagonal pattern
- Essentially one sheet of graphite, one atom thick
- sp² hybridized carbon atoms
- Two-dimensional material with delocalized π-electrons
Physical Properties:
- Strongest material known (200 times stronger than steel)
- Excellent electrical conductor
- Highest thermal conductivity
- Nearly transparent (97.7% light transmittance)
- Extremely flexible yet very stiff
- High electron mobility
Applications:
- Electronics (transistors, integrated circuits)
- Touchscreens and flexible displays
- Energy storage (supercapacitors, batteries)
- Composite materials for strength enhancement
- Sensors and biomedical applications
Exam Focus:
Andre Geim and Konstantin Novoselov received the 2010 Nobel Prize in Physics for their groundbreaking experiments with graphene. The isolation method involved using scotch tape to peel layers from graphite—a simple technique with revolutionary results.
5. Carbon Nanotubes (CNTs)

Single-walled carbon nanotube structure
Structure:
- Cylindrical structures formed by rolling graphene sheets
- Can be single-walled (SWCNT) or multi-walled (MWCNT)
- sp² hybridized carbon atoms arranged in hexagonal patterns
- Diameter: 0.4-100 nm; Length: up to several centimeters
- Different “chiralities” (armchair, zigzag, or chiral)
Physical Properties:
- Extremely high tensile strength (up to 100 times stronger than steel)
- Excellent electrical conductivity (can be metallic or semiconducting)
- Very high thermal conductivity
- Low density
- High aspect ratio (length to diameter ratio)
- Elastically flexible but resistant to torsion
Applications:
- Composite materials for strength enhancement
- Electronics (transistors, interconnects)
- Energy storage (batteries, supercapacitors)
- Biomedical applications (drug delivery, imaging)
- Sensors and field emission devices
Exam Focus:
The electrical properties of carbon nanotubes depend on their chirality—the exact way the graphene sheet is “rolled.” Armchair CNTs are always metallic, while zigzag and chiral ones can be either metallic or semiconducting.
6. Amorphous Carbon
Structure:
- No crystalline structure; random arrangement of carbon atoms
- Contains a mixture of sp², sp³, and sometimes sp hybridized carbon
- Examples include coal, soot, charcoal, and carbon black
- Contains various impurities depending on the source
Physical Properties:
- Black in color
- Variable hardness and density
- Moderate electrical conductivity
- Porous structure (especially in activated charcoal)
- High surface area
Applications:
- Fuel (coal)
- Filtration and purification (activated charcoal)
- Pigments (carbon black)
- Rubber reinforcement
- Adsorption of toxins and gases
Exam Focus:
Activated charcoal has exceptional adsorptive properties due to its high surface area (up to 3000 m²/g). This is why it’s used in filters, gas masks, and for treating poisoning cases.
Comparative Analysis of Carbon Allotropes
| Property | Diamond | Graphite | Fullerenes | Graphene |
|---|---|---|---|---|
| Hybridization | sp³ | sp² | sp² | sp² |
| Structure | Tetrahedral | Layered hexagonal | Spherical/cage-like | Single-layer hexagonal |
| Electrical conductivity | Insulator | Conductor | Semiconductor | Excellent conductor |
| Thermal conductivity | Very high | Anisotropic | Low | Highest known |
| Hardness | Highest (10) | Very soft (1-2) | Moderate | Very high |
| Density (g/cm³) | 3.5 | 2.2 | 1.65 (C₆₀) | 2.2 (theoretical) |
| Appearance | Transparent/colorless | Black, lustrous | Dark solid | Nearly transparent |
Key Exam Comparison Points:
When comparing allotropes in exams, focus on:
- How hybridization affects the physical structure
- How structure influences key properties (conductivity, hardness)
- The relationship between bonding patterns and practical applications
- The transition conditions between allotropes (high pressure, temperature)
Special Focus: Interconversion of Allotropes
Under specific conditions of temperature and pressure, carbon allotropes can be converted from one form to another:
- Graphite to Diamond: Requires very high pressure (>50,000 atmospheres) and high temperature (>1500°C)
- Diamond to Graphite: Thermodynamically favorable but kinetically slow at room temperature and pressure
- Graphite to Fullerenes: Can occur through electric arc discharge between graphite electrodes in an inert atmosphere
- Graphite to Graphene: Mechanical exfoliation, chemical vapor deposition, or chemical reduction methods
The positive enthalpy change indicates that diamond is less stable than graphite under standard conditions.
Exam Focus:
Although diamond has a higher enthalpy than graphite (is less thermodynamically stable), it does not spontaneously convert to graphite at room temperature due to an extremely high activation energy barrier. This is an excellent example of kinetic stability versus thermodynamic stability.
Practical Applications and Modern Research
Advanced Materials Science
Carbon allotropes form the foundation of many advanced materials:
- Carbon fiber: Lightweight, strong materials for aerospace, sports equipment
- Diamond-like carbon (DLC): Protective coatings with diamond-like properties
- Carbon nanotube composites: Ultra-strong, lightweight materials
- Graphene-enhanced polymers: Materials with improved electrical, thermal, and mechanical properties
Electronics and Energy
Carbon allotropes are revolutionizing electronics and energy storage:
- Graphene transistors: Faster, smaller, and more efficient than silicon
- Carbon nanotube memory: High-density, low-power data storage
- Graphene supercapacitors: Rapid charging, high capacity energy storage
- Carbon-based solar cells: Flexible, lightweight photovoltaics
Biomedical Applications
Carbon allotropes are finding increasing use in medicine:
- Fullerene-based drug delivery: Targeted therapy with reduced side effects
- Carbon nanotube biosensors: Ultra-sensitive detection of biomolecules
- Graphene-based tissue engineering: Scaffolds for cell growth and tissue regeneration
- Diamond-based implants: Biocompatible, durable medical devices
Exam Focus:
Questions on applications often ask you to connect the specific properties of an allotrope to its suitability for particular uses. For example, explain why graphite (not diamond) is used in electrodes based on its electrical conductivity properties.
Practice Questions for Exam Preparation
Multiple Choice Questions
-
Which carbon allotrope contains sp³ hybridized carbon atoms?
a) Graphite
b) Diamond
c) Fullerene
d) Graphene
Answer: b) Diamond
-
The electrical conductivity of graphite is due to:
a) Free carbon atoms
b) Delocalized electrons
c) Weak van der Waals forces
d) Strong covalent bonds
Answer: b) Delocalized electrons
-
Which allotrope of carbon has a soccer ball-like structure?
a) Diamond
b) Graphite
c) Buckminsterfullerene
d) Graphene
Answer: c) Buckminsterfullerene
Short Answer Questions
-
Explain why diamond is hard while graphite is soft, even though both are made entirely of carbon atoms.
Key points: Diamond has tetrahedral structure with strong covalent bonds in all directions; graphite has strong bonds within layers but weak forces between layers.
-
Why is graphite used as a lubricant despite having strong covalent bonds?
Key points: Weak van der Waals forces between layers allow them to slide past each other, creating lubricating effect.
-
Compare and contrast the structures of graphene and carbon nanotubes.
Key points: Graphene is 2D sheet of hexagonally arranged carbon atoms; carbon nanotubes are essentially rolled graphene sheets forming tubular structures.
Calculation Problem
Diamond has a density of 3.5 g/cm³, while graphite has a density of 2.2 g/cm³. Calculate the volume occupied by 1 mole of carbon atoms (12 g) in each form. What does this tell you about their structural arrangements?
Solution:
For diamond: V = m/ρ = 12 g ÷ 3.5 g/cm³ = 3.43 cm³
For graphite: V = m/ρ = 12 g ÷ 2.2 g/cm³ = 5.45 cm³
This indicates that graphite has a less compact structure than diamond, with more empty space between atoms, consistent with its layered structure.
Summary of Key Concepts
- Allotropy: Different structural forms of the same element in the same physical state
- Diamond: sp³ hybridized carbon in tetrahedral arrangement, extremely hard, electrical insulator
- Graphite: sp² hybridized carbon in layered hexagonal arrangement, soft, electrical conductor
- Fullerenes: sp² hybridized carbon in spherical/cage arrangements (C₆₀, C₇₀, etc.)
- Graphene: Single-layer of sp² hybridized carbon atoms, exceptional electrical and mechanical properties
- Carbon Nanotubes: Cylindrical structures of rolled graphene, excellent strength and electrical properties
- Amorphous Carbon: Random arrangement of carbon atoms with mixed hybridization
- Structure-Property Relationship: The physical and chemical properties of each allotrope directly relate to their atomic arrangement and bonding
References and Further Reading
- Atkins, P.W. and de Paula, J. (2017). Physical Chemistry, 11th Edition. Oxford University Press.
- Housecroft, C.E. and Sharpe, A.G. (2018). Inorganic Chemistry, 5th Edition. Pearson.
- Novoselov, K.S., et al. (2004). “Electric Field Effect in Atomically Thin Carbon Films.” Science, 306(5696), 666-669.
- O’Connell, M.J. (2018). Carbon Nanotubes: Properties and Applications. CRC Press.
- Kroto, H.W., et al. (1985). “C60: Buckminsterfullerene.” Nature, 318, 162-163.