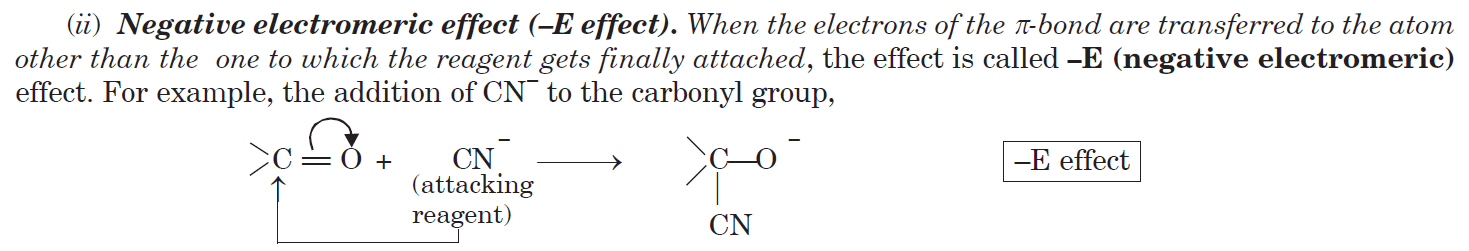Define Electromeric Effect: Definition and Examples
Understanding this temporary electronic effect in organic chemistry
The electromeric effect is a fundamental concept in organic chemistry that explains how π-electrons completely transfer during chemical reactions. For NEET, JEE, and CBSE Class 11-12 students, understanding this effect is crucial for predicting reaction mechanisms and product formation. Unlike permanent electronic effects, the electromeric effect is temporary and occurs only in the presence of a reagent.
Key Concept: The electromeric effect involves complete transfer of π-electrons to one atom in a multiple bond when attacked by a reagent.
Definition of Electromeric Effect
The electromeric effect (E effect) is defined as:
“The complete transfer of π-electrons from one atom to another in a multiple bond (double or triple bond) when attacked by a reagent, resulting in temporary polarization.”
This effect is observed only during chemical reactions and disappears when the attacking reagent is removed. It’s different from inductive and mesomeric effects which are permanent in nature.
Types of Electromeric Effect
+E Effect (Positive Electromeric Effect)
- π-electrons transfer toward the attacking reagent
- Occurs when the attacking species is electrophilic (electron-deficient)
- Example: Addition of H⁺ to C=C in alkenes
CH₂=CH₂ + H⁺ → CH₂⁺-CH₃ (π-electrons move toward H⁺)
-E Effect (Negative Electromeric Effect)
- π-electrons transfer away from the attacking reagent
- Occurs when the attacking species is nucleophilic (electron-rich)
- Example: Addition of CN⁻ to C=O in carbonyl compounds
CH₃CHO + CN⁻ → CH₃C⁻(OH)-CN (π-electrons move away from CN⁻)
Characteristics of Electromeric Effect
Key Features:
- Temporary effect – exists only during reaction
- Involves complete transfer of π-electrons (unlike partial transfer in mesomeric effect)
- Occurs in compounds with multiple bonds (C=C, C=O, C≡N etc.)
- Direction depends on nature of attacking reagent
- Helps explain addition reactions to unsaturated compounds
Examples in Organic Reactions
1. Addition to Alkenes
When HBr adds to ethene:
CH₂=CH₂ + HBr → CH₃-CH₂Br (π-electrons shift toward H⁺ first)
The +E effect creates a carbocation intermediate
2. Carbonyl Compounds
Nucleophilic addition to aldehydes:
CH₃CHO + HCN → CH₃CH(OH)CN (π-electrons shift toward O in first step)
The -E effect facilitates nucleophilic attack
Comparison with Other Electronic Effects
| Effect | Electromeric | Inductive | Mesomeric |
|---|---|---|---|
| Nature | Temporary | Permanent | Permanent |
| Electron Transfer | Complete | Partial | Partial |
| Bonds Involved | π-bonds only | σ-bonds | π-bonds |
| Occurrence | During reactions | Always present | Always present |
FAQs About Electromeric Effect
1. How is electromeric effect different from inductive effect?
The electromeric effect involves complete π-electron transfer during reactions (temporary), while inductive effect involves partial σ-electron displacement (permanent).
2. Does electromeric effect occur in single bonds?
No, it only occurs in multiple bonds (double/triple bonds) where π-electrons are available for complete transfer.
3. Why is electromeric effect temporary?
Because it only occurs when the molecule is under attack by a reagent and disappears when the reaction is complete.
4. Can both +E and -E effects occur in same molecule?
Yes, depending on whether the attacking reagent is electrophilic (+E) or nucleophilic (-E), but not simultaneously.
5. Is electromeric effect seen in benzene rings?
No, benzene shows resonance (mesomeric effect) but not electromeric effect as the π-electrons are delocalized, not completely transferred.
6. How does electromeric effect relate to Markovnikov’s rule?
The +E effect explains Markovnikov’s rule – π-electrons shift toward the electrophile (H⁺), creating a carbocation on the more substituted carbon.
7. Which is stronger: electromeric or mesomeric effect?
Mesomeric effect is generally stronger as it’s permanent, but electromeric effect is more significant during reactions.
8. Do all multiple bonds show electromeric effect?
Only when attacked by appropriate reagents – isolated multiple bonds without reagent attack don’t show this effect.
Summary of Key Points
- Electromeric effect is temporary complete π-electron transfer during reactions
- Two types: +E (toward electrophile) and -E (away from nucleophile)
- Occurs only in multiple bonds (C=C, C=O, C≡N etc.)
- Explains addition reaction mechanisms in unsaturated compounds
- Different from permanent effects like inductive and mesomeric effects
- Crucial for understanding Markovnikov’s rule and nucleophilic additions
Master More Organic Chemistry Concepts
Explore our guides on resonance, hyperconjugation, and reaction mechanisms!