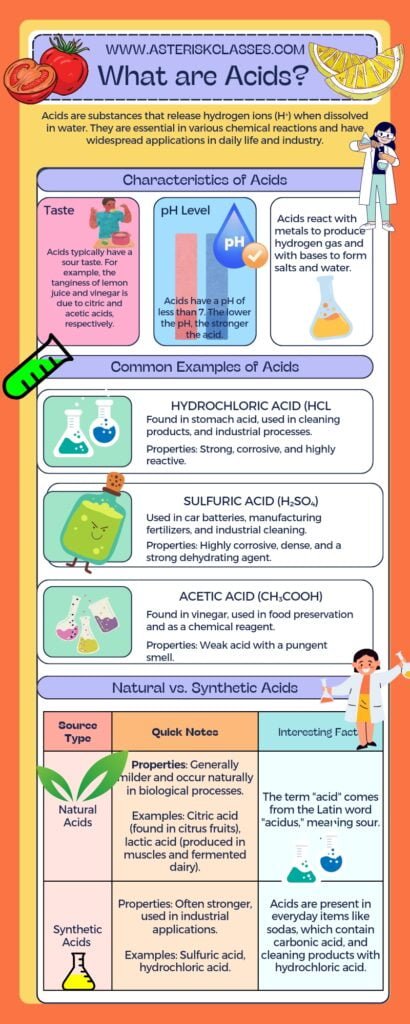
Acids are a fascinating and diverse group of substances with unique properties that make them vital to various scientific and industrial processes.
They are characterized by their sour taste, ability to turn blue litmus paper red, and their reactivity with metals to produce hydrogen gas.
Acids are also known to react with bases to form salts and water, a process known as neutralization.
There are two main types of acids: strong acids, which dissociate completely in water, such as hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3); and weak acids, which do not fully dissociate, including acetic acid (CH3COOH), carbonic acid (H2CO3), and citric acid (C6H8O7).
Examples of acids are abundant both in nature and in synthetic forms.
For instance, citric acid is found in citrus fruits, while industrial processes often use sulfuric acid due to its various applications.
Understanding the features and types of acids is crucial for their safe and effective use in everyday life and in specialized fields.