Acids, Bases and Salts Class 7th Science Chapter 4 of NCERT Book according to the Latest Syllabus Of JKBOSE and CBSE, NCERT Solutions.
 >
>I. Fill in the blank spaces by choosing correct words from the list given below:
List: bitter, ants, corrosive, citric, soapy, slaked lime.
- The acid found in lemons is __________ acid.
- Answer: citric
- The bases have a __________ taste and __________ touch.
- Answer: bitter, soapy
- The sting of the __________ contains formic acid.
- Answer: ants
- Sulphuric acid is highly __________ acid.
- Answer: corrosive
- Acidic soils are neutralized with __________.
- Answer: slaked lime
II. Statements given below are incorrect. Write the correct statements.
- Sulphuric acid is an example of an organic acid.
- Answer: Sulphuric acid is an example of a mineral acid.
- Blue litmus paper turns red in a basic solution.
- Answer: Blue litmus paper turns red in an acidic solution.
- China rose solution turns green in citric acid solution.
- Answer: China rose solution turns dark pink in citric acid solution.
- Formic acid is found in the sting of a grasshopper.
- Answer: Formic acid is found in the sting of ants.
- During neutralization, an acid reacts with a salt to form water and a base, as products.
- Answer: During neutralization, an acid reacts with a base to form water and a salt, as products.
III. Write true or false in front of the following statements:
- Tooth decay is caused by the presence of basic substance in the mouth.
- Answer: False
- The substance which shows different colours in acids and bases are called indicators.
- Answer: True
- Milk of magnesia and slaked lime are examples of neutral substances.
- Answer: False
- Acid rain is caused by the excess of carbon dioxide in air.
- Answer: False
- Potassium hydroxide turns blue litmus red.
- Answer: False
- Most of the fruits contain organic acids.
- Answer: True
Answer the following questions:
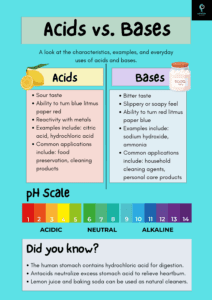
- Name three organic and three inorganic acids.
- Answer: Organic acids: Citric acid, Acetic acid, Formic acid. Inorganic acids: Hydrochloric acid, Sulphuric acid, Nitric acid.
- What are indicators? Name any three indicators and state the colour change which takes place in (i) acids (ii) bases.
- Answer: Indicators are substances that change color in the presence of an acid or a base. Examples:
- Litmus: Red in acid, Blue in base.
- Phenolphthalein: Colorless in acid, Pink in base.
- Methyl orange: Red in acid, Yellow in base.
- Answer: Indicators are substances that change color in the presence of an acid or a base. Examples:
- What are neutral substances? Give examples of two neutral substances.
- Answer: Neutral substances are neither acidic nor basic and do not change the color of indicators. Examples: Water, Table salt solution.
- You are given three unlabelled bottles A, B, and C, containing colorless solutions, such that one of them is acid, the other being basic and neutral. How will you distinguish between them by using china rose as an indicator?
- Answer: Add china rose solution to each bottle:
- Acidic solution will turn dark pink.
- Basic solution will turn green.
- Neutral solution will not change color.
- Answer: Add china rose solution to each bottle:
- What do you understand by the term neutralization? Describe an activity in which neutralization of hydrochloric acid takes place with sodium hydroxide, using phenolphthalein as an indicator.
- Answer: Neutralization is a chemical reaction where an acid and a base react to form water and a salt. Activity: Add phenolphthalein to hydrochloric acid (HCl), which remains colorless. Gradually add sodium hydroxide (NaOH) until the solution turns pink, indicating neutralization.
- Explain why:
- (a) Farmers add slaked lime to acidic soils.
- Answer: Farmers add slaked lime (calcium hydroxide) to neutralize the acidity of the soil, improving its fertility.
- (b) Soap solution or baking soda paste is applied on the part of the body stung by a bee.
- Answer: Bee stings are acidic; applying a basic substance like baking soda neutralizes the acid, relieving pain and inflammation.
- (c) Factory wastes and city sewerage is neutralized before discharging in rivers.
- Answer: Neutralizing acidic or basic factory wastes prevents harm to aquatic life and maintains the pH balance of the river water.
- (d) Antacids are used for relieving stomach activity.
- Answer: Antacids neutralize excess stomach acid, relieving indigestion and heartburn.
- (a) Farmers add slaked lime to acidic soils.
- Give four differences between the acids and the alkalis.
- Answer:
- Acids taste sour, alkalis taste bitter.
- Acids turn blue litmus red, alkalis turn red litmus blue.
- Acids have a pH less than 7, alkalis have a pH more than 7.
- Acids can corrode metals, alkalis feel slippery.
- Answer:
- Write an equation when hydrochloric acid neutralizes sodium hydroxide.
- Answer: HCl + NaOH → NaCl + H₂O
MCQs: Choose the correct answer
- Name the acid which is present in the sting of ants.
- (a) Acetic acid
- (b) Formic acid
- (c) Oxalic acid
- (d) Tartaric acid
- Answer: (b) Formic acid
- Blue litmus turns red in which solution:
- (a) Acidic solution
- (b) Basic solution
- (c) Both acidic and basic solutions
- (d) Neutral solution
- Answer: (a) Acidic solution
- Which of the following is not a mineral acid?
- (a) Hydrochloric acid
- (b) Sulphuric acid
- (c) Citric acid
- (d) Nitric acid
- Answer: (c) Citric acid
- When few drops of china rose solution is added to shampoo taken in test tube the colour of the solution becomes:
- (a) Blue
- (b) Red
- (c) Green
- (d) Deep Pink
- Answer: (c) Green

