Understanding Electron Dot Structures: Electron dot structure of co2
A comprehensive guide to Lewis structures in chemistry
Introduction to Electron Dot Structures
Electron dot structures, also known as Lewis structures, are fundamental diagrams in chemistry that illustrate the bonding between atoms in molecules and the lone pairs of electrons that may exist. Named after Gilbert N. Lewis, these structures provide invaluable insights into molecular geometry, chemical bonding, and reactivity patterns.
In these representations, valence electrons are depicted as dots around atomic symbols, while bonded electron pairs are typically shown as lines connecting atoms. This visual approach enables chemists to predict molecular behavior, understand reaction mechanisms, and design new compounds with desired properties.
Key Principles of Electron Dot Structures
- Atoms share electrons to achieve a stable outer shell configuration (typically 8 electrons – the octet rule)
- Dots represent valence (outer shell) electrons available for bonding
- Lines represent shared pairs of electrons forming chemical bonds
- Single bonds use 2 electrons, double bonds use 4, and triple bonds use 6
- Lone pairs are non-bonding electron pairs that influence molecular geometry
Electron Dot Structures Diagram
Visual representation of all nine compounds covered in this guide:
Electron Dot Structures Diagram
Replace this placeholder with your electron dot structures image URL:
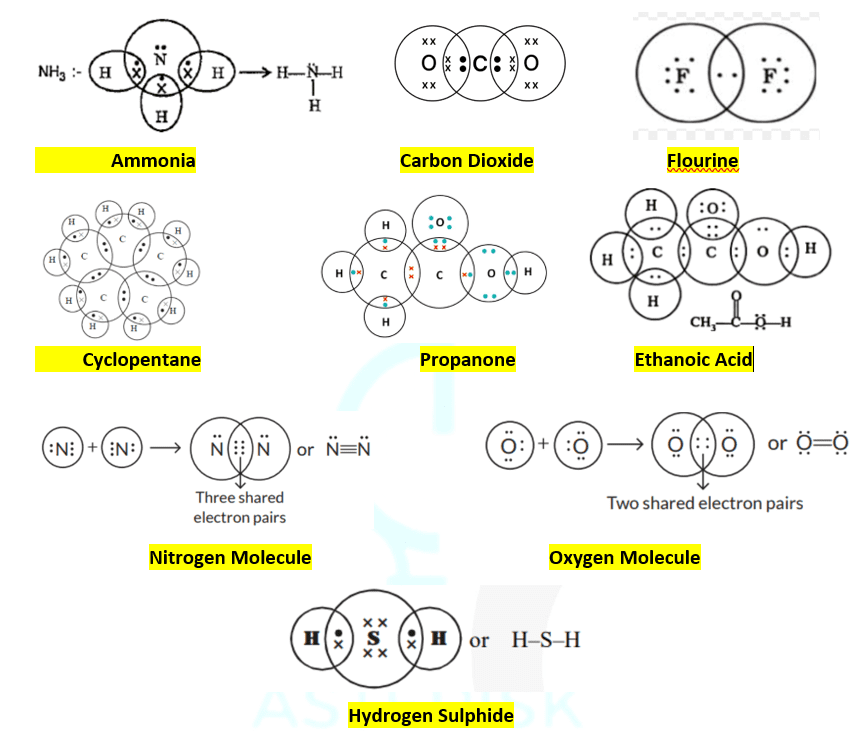
The diagram above shows Lewis structures for CO₂, CH₃COOH, C₅H₁₀, CH₃COCH₃, NH₃, H₂S, F₂, O₂, and N₂
Detailed Analysis of Compounds
CO₂
Carbon Dioxide
Structure: Linear molecule
Bonds: Two double bonds
Geometry: 180° bond angle
Description: Carbon forms double bonds with each oxygen atom. Each oxygen has two lone pairs, satisfying the octet rule for all atoms.
CH₃COOH
Ethanoic Acid
Structure: Carboxylic acid
Bonds: Mix of and
Groups: Methyl (-CH₃) + Carboxyl (-COOH)
Description: Contains a carbonyl group (C=O) and hydroxyl group (-OH). The carbonyl oxygen has two lone pairs.
C₅H₁₀
Cyclopentane
Structure: Five-membered ring
Bonds: All single bonds
Geometry: Pentagon-shaped
Description: Cyclic alkane with each carbon bonded to two other carbons and two hydrogens, forming a stable ring structure.
CH₃COCH₃
Propanone (Acetone)
Structure: Ketone
Bonds: at carbonyl +
Groups: Two methyl groups + carbonyl
Description: Symmetrical ketone with a central carbonyl group flanked by two methyl groups. Oxygen has two lone pairs.
NH₃
Ammonia
Structure: Trigonal pyramidal
Bonds: Three bonds
Lone pairs: One on nitrogen
Description: Nitrogen bonded to three hydrogens with one lone pair, creating a pyramidal molecular geometry.
H₂S
Hydrogen Sulfide
Structure: Bent/Angular
Bonds: Two bonds
Lone pairs: Two on sulfur
Description: Sulfur bonded to two hydrogens with two lone pairs, resulting in a bent molecular geometry similar to water.
F₂
Fluorine
Structure: Diatomic molecule
Bonds: One bond
Lone pairs: Three on each F
Description: Two fluorine atoms sharing one electron pair, with each fluorine having three lone pairs to complete their octets.
O₂
Oxygen
Structure: Diatomic molecule
Bonds: One bond
Lone pairs: Two on each O
Description: Two oxygen atoms sharing two electron pairs in a double bond, with each oxygen having two lone pairs.
N₂
Nitrogen
Structure: Diatomic molecule
Bonds: One bond
Lone pairs: One on each N
Description: Two nitrogen atoms sharing three electron pairs in a very strong triple bond, with each nitrogen having one lone pair.
Applications and Importance
Predicting Molecular Geometry
Lewis structures help determine the three-dimensional arrangement of atoms in molecules using VSEPR theory.
Understanding Polarity
Electron distribution patterns reveal whether molecules are polar or nonpolar, affecting their physical properties.
Predicting Reactivity
Lone pairs and bond types indicate reactive sites and help predict chemical behavior and reaction mechanisms.
Visualizing Bonding
Lewis structures provide clear visual representations of how atoms share electrons to form stable compounds.
Limitations of Lewis Structures
While Lewis structures are invaluable tools, they have important limitations:
- • They don’t account for molecular orbital theory or electron delocalization
- • Resonance structures may be needed for accurate representation
- • They don’t show relative bond strengths or bond lengths accurately
- • Advanced bonding concepts require quantum mechanical models
Conclusion
Electron dot structures serve as fundamental tools in chemistry education and research, providing clear visual representations of molecular bonding and electron distribution. Through the nine compounds examined in this guide—from simple diatomic molecules like N₂ and O₂ to more complex organic compounds like ethanoic acid and propanone—we can see how Lewis structures reveal important insights about molecular behavior.
Understanding these structures enables chemists to predict molecular geometry, polarity, and reactivity patterns, forming the foundation for more advanced concepts in organic chemistry, biochemistry, and materials science. While Lewis structures have limitations and may require supplementation with more sophisticated models, they remain indispensable for developing chemical intuition and solving practical problems.
Key Takeaways
- • Lewis structures visualize valence electrons and chemical bonding patterns
- • Different bond types (single, double, triple) involve different numbers of shared electrons
- • Lone pairs significantly influence molecular geometry and chemical properties
- • These structures help predict molecular behavior and reaction pathways
- • Understanding electron distribution is crucial for advanced chemistry concepts


