Extraction of Metals class 10 in Accordance with Activity Series
Understanding how the reactivity of metals determines their extraction methods
Introduction
The extraction of metals from their ores is a critical process in metallurgy. The method used to extract a metal depends significantly on its position in the reactivity series. This relationship between a metal’s reactivity and its extraction method is fundamental to understanding metallurgical processes and is often tested in chemistry examinations.
Key Concept
The more reactive a metal is, the more difficult it is to extract from its compounds. Consequently, different extraction techniques are applied based on where a metal stands in the reactivity series.
Quick Recap: The Reactivity Series
Before diving into extraction methods, let’s recall the reactivity series of metals (from most reactive to least reactive):
| Reactivity Level | Metals | General Properties |
|---|---|---|
| Most Reactive | Potassium (K) Sodium (Na) Lithium (Li) Calcium (Ca) |
React vigorously with water and oxygen |
| Very Reactive | Magnesium (Mg) Aluminum (Al) |
React with cold water (Mg slowly), steam, and dilute acids |
| Moderately Reactive | Zinc (Zn) Iron (Fe) Tin (Sn) Lead (Pb) |
React with steam and acids but not with cold water |
| Least Reactive | Copper (Cu) Silver (Ag) Gold (Au) Platinum (Pt) |
Do not react with water or dilute acids |
Extraction Methods Based on Reactivity
The extraction method for a metal directly correlates with its position in the reactivity series. We can classify these methods into three main categories:
1. Electrolysis (Highly Reactive Metals)
Metals at the top of the reactivity series (K, Na, Li, Ca, Mg, Al) are too reactive to be extracted by chemical reduction methods. These metals are extracted using electrolysis of their molten compounds.
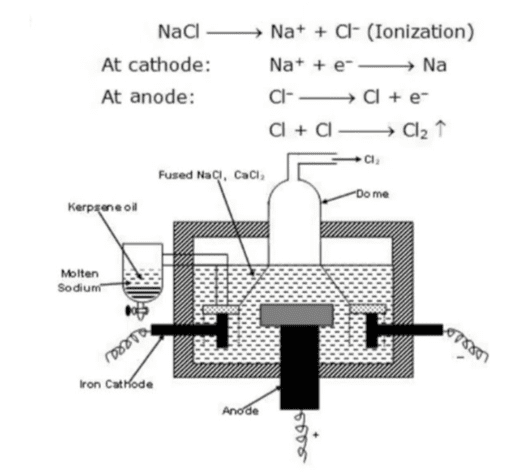
Example: Sodium Extraction
Sodium is extracted by the electrolysis of molten sodium chloride (NaCl). Since solid NaCl cannot conduct electricity, it is first melted (at around 800°C) before electrolysis.
At the cathode: Na+ + e– → Na (sodium metal is formed)
At the anode: 2Cl– → Cl2 + 2e– (chlorine gas is released)
Overall reaction: 2NaCl → 2Na + Cl2
Example: Aluminum Extraction
Aluminum is extracted by the electrolysis of alumina (Al2O3) dissolved in molten cryolite (Na3AlF6). Cryolite lowers the melting point of alumina and improves electrical conductivity.
At the cathode: Al3+ + 3e– → Al (aluminum metal is formed)
At the anode: 2O2- → O2 + 4e– (oxygen gas is released)
Overall reaction: 2Al2O3 → 4Al + 3O2
2. Chemical Reduction (Moderately Reactive Metals)
Metals in the middle of the reactivity series (Zn, Fe, Pb, etc.) are extracted by chemical reduction, typically using carbon (as coke) or carbon monoxide as reducing agents.
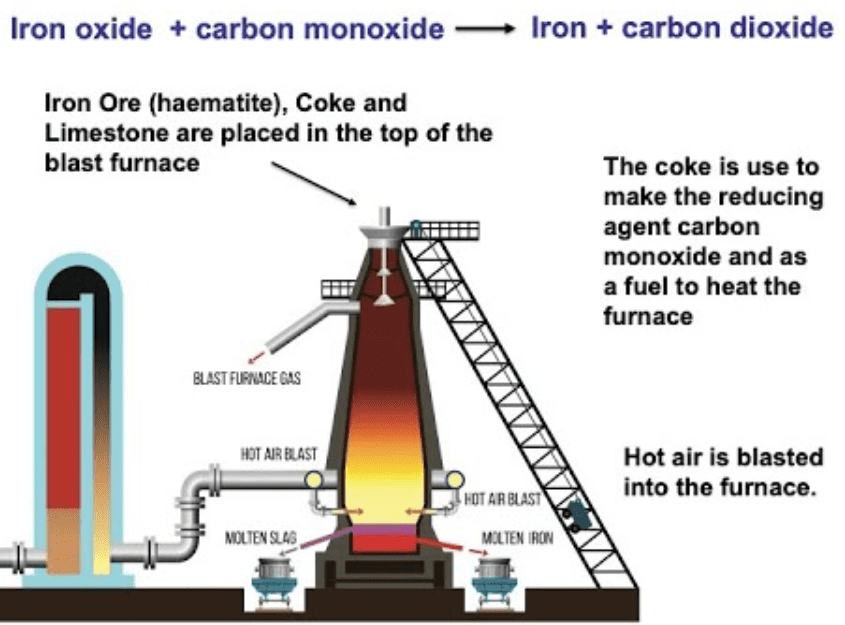
Example: Iron Extraction from Hematite
Iron is extracted from hematite (Fe2O3) in a blast furnace using coke (carbon) as a reducing agent.
Fe2O3 + 3C → 2Fe + 3CO
Fe2O3 + 3CO → 2Fe + 3CO2
The reducing agent (carbon) is more reactive than iron, enabling it to remove oxygen from iron oxide.
Example: Zinc Extraction
Zinc is extracted from zinc oxide (ZnO) by reduction with carbon.
ZnO + C → Zn + CO
Since zinc is less reactive than carbon at high temperatures, carbon can reduce zinc oxide to zinc metal.
3. Self-Reduction or Physical Methods (Least Reactive Metals)
Metals at the bottom of the reactivity series (Cu, Ag, Au, Pt) are often found in their native state or can be easily extracted by heating their oxides in air or through simple displacement reactions.
Example: Copper Extraction
Copper can be extracted by self-reduction of copper(I) sulfide during the smelting process:
2Cu2S + 3O2 → 2Cu2O + 2SO2
2Cu2O + Cu2S → 6Cu + SO2
Alternatively, copper oxides can be directly reduced with carbon:
CuO + C → Cu + CO
Example: Silver Extraction
Silver is often extracted by treating silver ores with cyanide solution in the presence of oxygen, followed by displacement with zinc:
4Ag + 8NaCN + O2 + 2H2O → 4Na[Ag(CN)2] + 4NaOH
2Na[Ag(CN)2] + Zn → Na2[Zn(CN)4] + 2Ag
Since zinc is more reactive than silver, it displaces silver from its solution.
Summary Table: Metal Extraction Based on Reactivity
| Reactivity Level | Metals | Extraction Method | Example |
|---|---|---|---|
| High (K, Na, Ca, Mg, Al) | Potassium, Sodium, Calcium, Magnesium, Aluminum | Electrolysis of molten compounds | Na from molten NaCl, Al from Al2O3 in cryolite |
| Medium (Zn, Fe, Pb, Sn) | Zinc, Iron, Lead, Tin | Chemical reduction with carbon or carbon monoxide | Fe from Fe2O3 using carbon in a blast furnace |
| Low (Cu, Hg, Ag, Au) | Copper, Mercury, Silver, Gold | Simple heating, self-reduction, or displacement reactions | Cu from Cu2S by partial roasting and smelting |
Exam Tips and Key Points to Remember
Exam Focus
- Correlation Rule: The more reactive a metal, the more difficult it is to extract.
- Method Selection: Be able to predict the appropriate extraction method based on a metal’s position in the reactivity series.
- Electrolysis Applications: Remember that only very reactive metals require electrolysis for extraction.
- Reduction Agents: Understand that carbon can only reduce metal oxides of metals less reactive than carbon.
- Hydrogen Reduction: Metals like copper and silver can be reduced by hydrogen, but more reactive metals like iron cannot.
- Native State: The least reactive metals often occur in their native (elemental) state in nature.
Practice Questions
Test Your Understanding
- Why is carbon not used to extract sodium from sodium oxide?
- Explain why electrolysis is necessary for aluminum extraction but not for copper extraction.
- Which extraction method would be most appropriate for extracting magnesium from magnesium chloride? Justify your answer.
- Iron is extracted using carbon as a reducing agent. Would the same method work for extracting potassium? Explain.
- Arrange the following metals in order of increasing difficulty of extraction: copper, sodium, iron, gold.
Answers
- Carbon cannot be used to extract sodium from sodium oxide because sodium is more reactive than carbon. Carbon can only reduce oxides of metals that are less reactive than itself.
- Aluminum is highly reactive and forms strong bonds with oxygen that cannot be broken by chemical reduction with carbon. Therefore, electrolysis is necessary. Copper is less reactive, so its oxide can be reduced by heating with carbon or even by self-reduction during smelting.
- Electrolysis would be most appropriate for magnesium chloride because magnesium is a highly reactive metal. Chemical reduction would not be effective as magnesium is more reactive than common reducing agents like carbon.
- No, the same method would not work for potassium because potassium is much more reactive than iron and is located above carbon in the reactivity series. Carbon cannot reduce potassium compounds.
- Gold (easiest to extract) → Copper → Iron → Sodium (most difficult to extract). This order follows the reactivity series from least reactive to most reactive.



