Helium – The Noble Gas with Surprising Uses
Introduction
Helium, symbolized as He with an atomic number of 2, is a noble gas that stands out for its unique properties and fascinating applications. As the second lightest and second most abundant element in the universe, helium plays a vital role in various scientific, industrial, and recreational fields.
History and Discovery
Helium was first discovered in 1868 during a solar eclipse by French astronomer Pierre Janssen and English astronomer Norman Lockyer. They observed a yellow spectral line in the Sun’s light, which could not be attributed to any known element at the time. The element was named “helium” after the Greek word “Helios”, meaning Sun.
Fascinating Facts about Helium
- Helium is the only element that remains in a liquid state at absolute zero under standard pressure.
- It is completely inert and does not form compounds under normal conditions.
- Helium is used to inflate balloons because it is lighter than air and non-flammable, unlike hydrogen.
- It has the lowest boiling and melting points of any element (-268.93°C and -272.20°C, respectively).
- Helium is the second most abundant element in the universe, formed during the Big Bang.
Properties of Helium
Physical Properties
- State: Colorless, odorless, and tasteless gas.
- Density: Much lighter than air, making it ideal for lifting applications.
- Thermal Conductivity: Excellent thermal conductor, used in cooling systems.
Chemical Properties
Helium is an inert gas, meaning it does not readily react with other elements or compounds. Its outermost electron shell is full, making it highly stable and non-reactive.
Applications of Helium
Despite its inertness, helium has a wide range of uses, including:
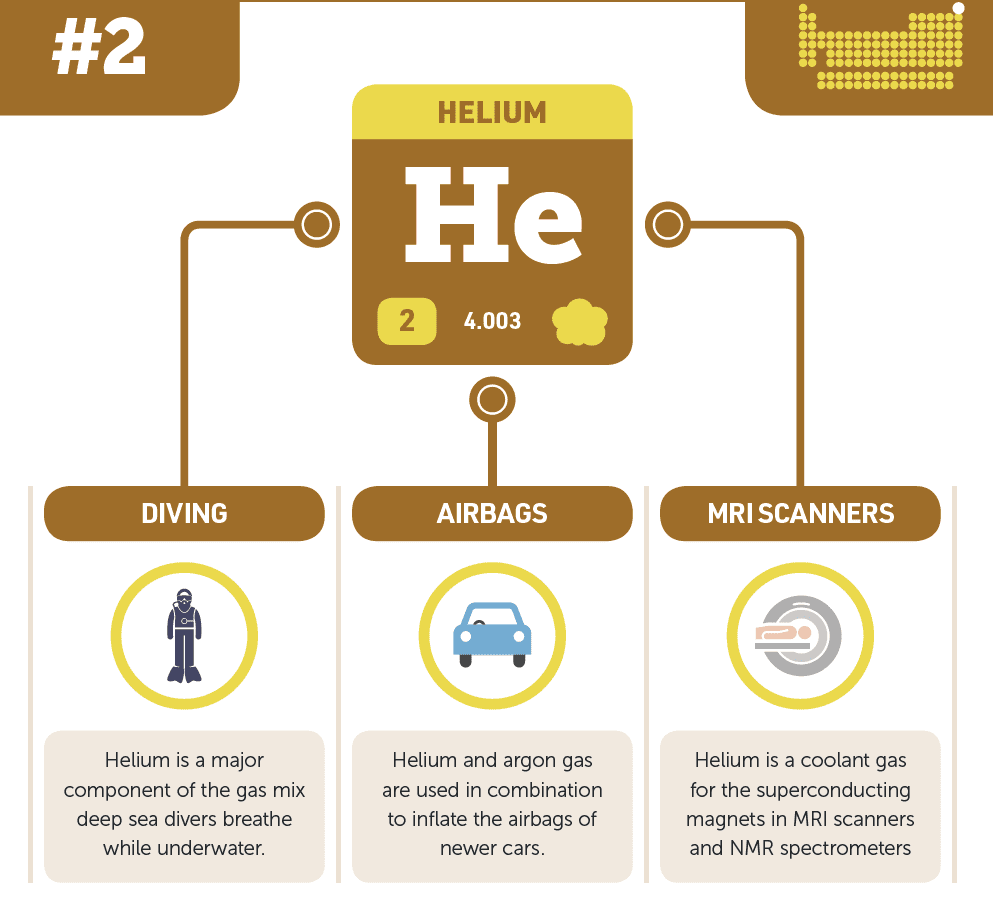
- Medical: Used in MRI scanners and breathing mixtures for deep-sea divers.
- Aerospace: Helps pressurize and purge fuel tanks in rockets.
- Science: Essential for cryogenics and supercooling superconductors.
- Entertainment: Inflating party balloons and blimps.
- Welding: Acts as a shielding gas in arc welding processes.
Conclusion
Helium is more than just a gas that makes balloons float; it is a critical resource for modern science, medicine, and industry. With its fascinating properties and diverse applications, helium continues to be an element of great significance in our daily lives and beyond.

