Brief History
In 1911, Ernest Rutherford, a New Zealand physicist, proposed the Rutherford model of the atom. He is renowned for his Gold Foil Experiment, which led to the discovery of the atomic nucleus. His model was a departure from J.J. Thomson’s “plum pudding” model and laid the groundwork for the modern understanding of atomic structure.
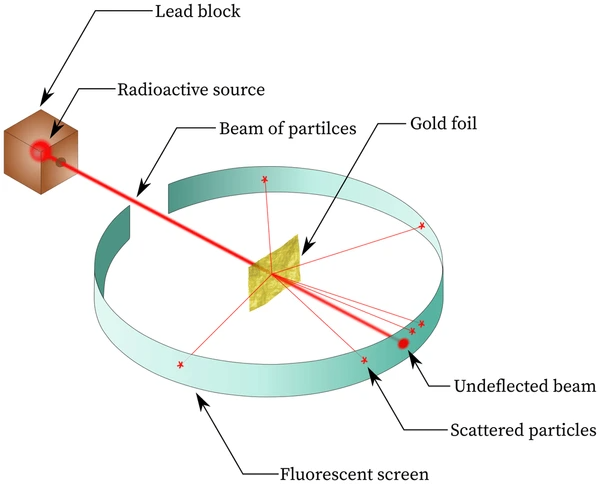 >
>The Gold Foil Experiment
In the experiment, Rutherford bombarded a thin sheet of gold foil with alpha particles (positively charged helium nuclei). Most of the particles passed through, but some were deflected, and a few even bounced back. This unexpected result led to a significant conclusion about the structure of the atom.
Postulates of Rutherford’s Atomic Model
- An atom consists mostly of empty space.
- There is a tiny, dense, and positively charged nucleus at the center of the atom.
- Electrons orbit the nucleus in circular paths.
- The nucleus contains almost all of the atom’s mass.
Limitations of Rutherford’s Model
- Rutherford’s model could not explain the stability of the atom, as classical physics suggested that orbiting electrons should lose energy and spiral into the nucleus.
- The model did not describe the arrangement of electrons around the nucleus, nor the chemical properties of elements.
- It could not explain atomic spectra, especially the discrete lines observed in the hydrogen spectrum.
Frequently Asked Questions (FAQs)
1. Who proposed the Rutherford atomic model?
Ernest Rutherford proposed the model in 1911.
2. What experiment led to Rutherford’s model?
The Gold Foil Experiment, in which alpha particles were directed at a thin gold foil, led to the development of his atomic model.
3. What did Rutherford discover?
Rutherford discovered that the atom has a small, dense nucleus where most of its mass is concentrated.
4. What is the significance of the Gold Foil Experiment?
The experiment demonstrated that atoms consist mainly of empty space and have a central nucleus, disproving earlier atomic models like Thomson’s plum pudding model.
5. What is the nucleus according to Rutherford?
The nucleus is a small, dense region at the center of the atom, which contains all of the atom’s positive charge and most of its mass.
6. Why was Rutherford’s model considered incomplete?
Rutherford’s model failed to explain why electrons did not spiral into the nucleus, as predicted by classical physics.
7. What are the limitations of the Rutherford model?
It could not explain the stability of atoms, atomic spectra, or the arrangement of electrons.
8. How did Rutherford’s model differ from Thomson’s model?
Thomson’s model depicted electrons embedded within a positively charged “pudding,” while Rutherford’s model placed electrons in orbit around a central nucleus.
9. How did Rutherford’s experiment challenge classical physics?
Classical physics suggested that orbiting electrons would lose energy and collapse into the nucleus, but this did not happen, posing a significant challenge to the theory.
10. What experiment succeeded Rutherford’s model?
Rutherford’s model was succeeded by Niels Bohr’s model, which incorporated quantum theory to explain atomic stability and electron energy levels.
Thompson model of Atom: Here