Introduction to Matter in Our Surroundings
Matter is anything that occupies space and has mass. From the air we breathe to the food we eat, everything around us is composed of matter. Understanding matter and its properties helps us comprehend the physical world and the various changes it undergoes.
Physical Nature of Matter
How Small Are These Particles?
Matter is composed of tiny particles that are too small to be seen with the naked eye. These particles are constantly moving and have spaces between them.
Example: When you dissolve a small amount of potassium permanganate in water, the entire solution becomes uniformly purple, indicating the presence of very small particles.
Characteristics of Particles of Matter
Particles of Matter Have Space Between Them
Particles of matter are not tightly packed but have spaces between them. The amount of space determines the state of matter.
Example: When sugar dissolves in water, it occupies the spaces between the water particles.
Particles of Matter Attract Each Other
Particles of matter attract each other with varying strengths, which influences their physical properties.
Example: Solids have strong attractive forces between particles, giving them a definite shape and volume.
States of Matter
Matter exists in three primary states: solid, liquid, and gas. Each state has distinct characteristics based on particle arrangement and movement.
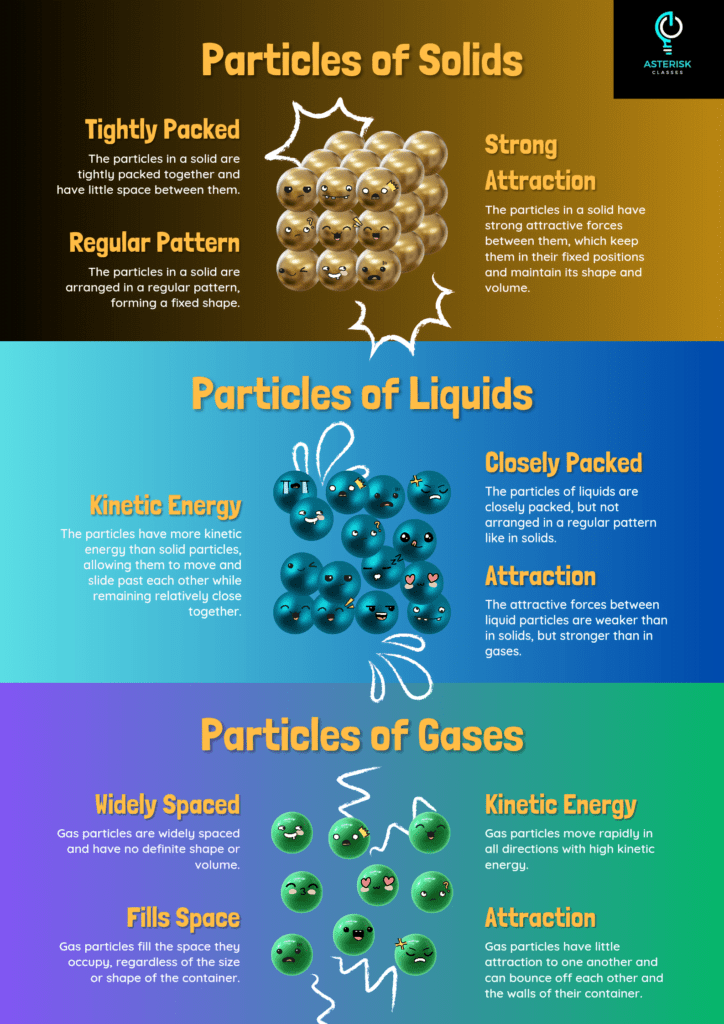 >
>Solid State
- Characteristics: Definite shape and volume. Particles are closely packed in a fixed arrangement and can only vibrate in place.
- Example: Ice, wood, and metal.
Liquid State
- Characteristics: Definite volume but takes the shape of its container. Particles are closely packed but can move past each other, allowing liquids to flow.
- Example: Water, milk, and oil.
Gaseous State
- Characteristics: Neither a definite shape nor a definite volume. Particles are far apart and move freely, filling any container.
- Example: Air, oxygen, and carbon dioxide.
Can Matter Change Its State?
Matter can change from one state to another when conditions such as temperature and pressure change. These changes are physical and reversible.
Effect of Change of Temperature
Temperature affects the kinetic energy of particles, causing changes in the state of matter.
- Latent Heat: Heat required to change the state of matter without changing its temperature.
- Latent Heat of Fusion: Heat required to change a solid into a liquid at its melting point.
- Example: Melting ice to water.
- Latent Heat of Vaporisation: Heat required to change a liquid into a gas at its boiling point.
- Example: Boiling water to steam.
Effect of Change of Pressure
Pressure can also cause changes in the state of matter. Increasing pressure on a gas can compress it into a liquid, and decreasing pressure can allow a liquid to become a gas.
Example: Carbon dioxide gas can be compressed into liquid CO₂ in fire extinguishers.
Evaporation
Evaporation is the process where liquid changes into gas at a temperature below its boiling point. This process occurs at the surface of the liquid.
Factors Affecting Evaporation
- Surface Area: Larger surface area increases evaporation.
- Temperature: Higher temperatures increase the rate of evaporation.
- Humidity: Lower humidity levels increase evaporation.
- Wind Speed: Higher wind speeds increase evaporation.
How Does Evaporation Cause Cooling?
During evaporation, the liquid particles absorb energy from their surroundings to change into gas, causing the surroundings to cool.
Example: Sweating cools the body as the sweat evaporates, absorbing heat from the skin.
Why Do We Wear Cotton Clothes During Summer?
Cotton is a good absorber of water. It absorbs sweat from our body and exposes it to the atmosphere for easy evaporation. This helps in cooling our body.
Drops on the Outer Surface of Ice-Cold Container
When a cold object is placed in a warm environment, water vapor from the air condenses on the surface of the object, forming water droplets.
Example: Drops of water form on the outside of a cold glass of water due to condensation of humid air.
Conclusion
Understanding these fundamental concepts of matter and its properties provides a foundation for exploring the fascinating world of chemistry and the behavior of materials in different conditions. Whether it’s the solid ice in your drink, the liquid water you drink, or the gaseous air you breathe, the states of matter and their transformations are all around us, shaping our everyday experiences.