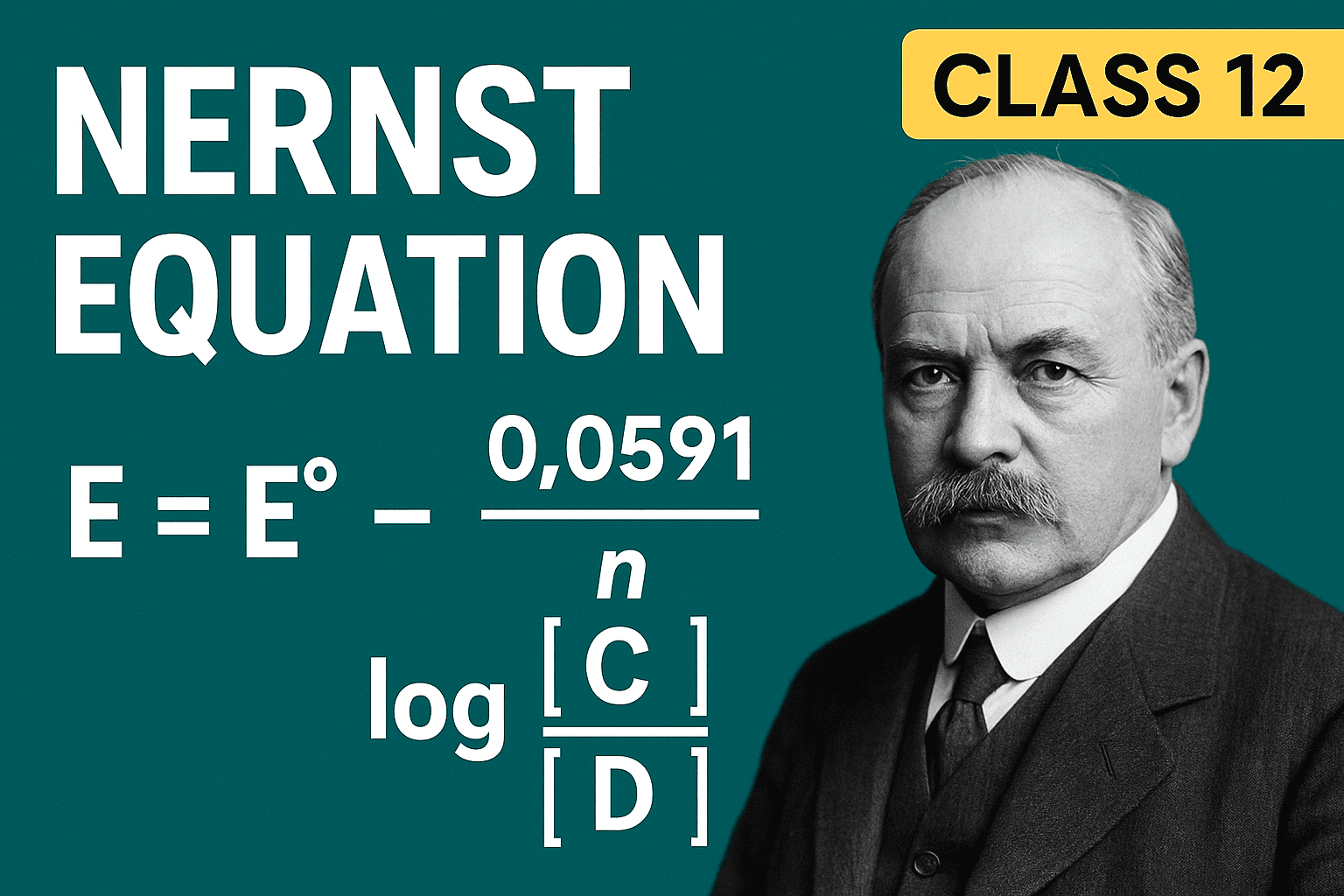Nernst Equation derivation class 12
Complete Derivation with 10 Numerical Problems
Electrochemistry | Physical Chemistry
Nernst Equation Derivation
Definition:
The Nernst equation relates the reduction potential of an electrochemical reaction to the standard electrode potential, temperature, and activities of the chemical species involved.
Derivation Steps:
Start with Gibbs free energy change: ΔG = ΔG° + RT lnQ
Relate to cell potential: ΔG = -nFE and ΔG° = -nFE°
Substitute: -nFE = -nFE° + RT lnQ
Divide by -nF: E = E° – (RT/nF) lnQ
At 298K, convert to log10: E = E° – (0.0592/n) logQ
Nernst Equation (General Form)
E = E° – (RT/nF) lnQ
At 298K (25°C):
E = E° – (0.0592/n) logQ
Where:
- E = Cell potential
- E° = Standard cell potential
- R = Universal gas constant (8.314 J/mol·K)
- T = Temperature (K)
- n = Number of electrons transferred
- F = Faraday’s constant (96485 C/mol)
- Q = Reaction quotient
Key Points:
- Applies to both galvanic and electrolytic cells
- Shows how potential changes with concentration
- At equilibrium (E=0), Q becomes equilibrium constant K
- For pure solids/liquids, activity = 1
- For gases, use partial pressures
Nernst Equation Numerical Problems
1. Calculate the cell potential at 25°C for: Zn|Zn²⁺(0.1M)||Cu²⁺(0.01M)|Cu (Given E° = 1.10V)
Solution:
Cell reaction: Zn + Cu²⁺ → Zn²⁺ + Cu (n=2)
Q = [Zn²⁺]/[Cu²⁺] = 0.1/0.01 = 10
E = E° – (0.0592/n) logQ
E = 1.10 – (0.0592/2) log10
E = 1.10 – 0.0296 = 1.0704 V
2. Find the potential of a hydrogen electrode in contact with a solution of pH = 3 at 25°C.
Solution:
For SHE: 2H⁺ + 2e⁻ → H₂ (E°=0V, n=2)
pH=3 ⇒ [H⁺] = 10⁻³ M
Q = 1/[H⁺]² = 1/(10⁻³)² = 10⁶
E = 0 – (0.0592/2) log(10⁶)
E = -0.0296 × 6 = -0.1776 V
3. Calculate EMF of the cell: Ni|Ni²⁺(0.1M)||Ag⁺(0.001M)|Ag at 25°C (Given E°Ni²⁺/Ni = -0.25V, E°Ag⁺/Ag = 0.80V)
Solution:
E°cell = 0.80 – (-0.25) = 1.05V
Cell reaction: Ni + 2Ag⁺ → Ni²⁺ + 2Ag (n=2)
Q = [Ni²⁺]/[Ag⁺]² = 0.1/(0.001)² = 10⁵
E = 1.05 – (0.0592/2) log(10⁵)
E = 1.05 – 0.148 = 0.902 V
4. What is the concentration of Cu²⁺ when the cell Zn|Zn²⁺(1M)||Cu²⁺(xM)|Cu has EMF = 1.05V at 25°C? (E° = 1.10V)
Solution:
1.05 = 1.10 – (0.0592/2) log(1/x)
-0.05 = -0.0296 log(1/x)
log(1/x) = 1.689 ⇒ 1/x = 101.689 ≈ 48.9
x = 1/48.9 ≈ 0.0204 M
5. Calculate the equilibrium constant for the reaction: Fe²⁺ + Ag⁺ ⇌ Fe³⁺ + Ag at 25°C (Given E°Fe³⁺/Fe²⁺ = 0.77V, E°Ag⁺/Ag = 0.80V)
Solution:
E°cell = 0.80 – 0.77 = 0.03V (n=1)
At equilibrium, E=0 ⇒ 0 = E° – (0.0592/n) logK
logK = nE°/0.0592 = (1×0.03)/0.0592 ≈ 0.5068
K = 100.5068 ≈ 3.21
6. Find the pH of a solution in contact with hydrogen electrode that shows potential of -0.118V at 25°C vs SHE.
Solution:
-0.118 = 0 – (0.0592/2) log(1/[H⁺]²)
-0.118 = -0.0592 log(1/[H⁺])
log(1/[H⁺]) = 2 ⇒ pH = 2
7. Calculate the potential of a Daniel cell at 25°C when [Zn²⁺] = 0.5M and [Cu²⁺] = 0.01M (E° = 1.10V)
Solution:
Q = [Zn²⁺]/[Cu²⁺] = 0.5/0.01 = 50
E = 1.10 – (0.0592/2) log50
log50 ≈ 1.699 ⇒ E = 1.10 – 0.0296×1.699
E ≈ 1.10 – 0.0503 ≈ 1.0497 V
8. What temperature would result in a cell potential of 1.08V for Zn|Zn²⁺(1M)||Cu²⁺(0.1M)|Cu if at 25°C its EMF is 1.07V (E° = 1.10V)?
Solution:
First find Q = 1/0.1 = 10
At 25°C: 1.07=1.10-(0.0592/2)log10 (verification)
General form: 1.08=1.10-(RT/2F)ln10
-0.02 = -(RT/2F)×2.303 ⇒ T = 0.04F/(2.303R)
T ≈ 0.04×96485/(2.303×8.314) ≈ 201.5K (-71.5°C)
9. Calculate the silver ion concentration in a cell Ag|Ag⁺(xM)||H⁺(1M)|H₂(1atm)|Pt that has EMF = 0.80V at 25°C (E°Ag⁺/Ag = 0.80V)
Solution:
0.80 = 0.80 – (0.0592/1) log(1/[Ag⁺])
0 = log(1/[Ag⁺]) ⇒ 1/[Ag⁺] = 10⁰ = 1
[Ag⁺] = 1 M
10. Find the potential of: Pt|Fe²⁺(0.1M),Fe³⁺(0.01M)||MnO₄⁻(0.05M),Mn²⁺(0.1M),H⁺(1M)|Pt at 25°C (Given E°MnO₄⁻/Mn²⁺ = 1.51V, E°Fe³⁺/Fe²⁺ = 0.77V)
Solution:
Balance redox: MnO₄⁻ + 5Fe²⁺ + 8H⁺ → Mn²⁺ + 5Fe³⁺ + 4H₂O (n=5)
E°cell = 1.51 – 0.77 = 0.74V
Q = [Mn²⁺][Fe³⁺]⁵/([MnO₄⁻][Fe²⁺]⁵[H⁺]⁸)
Q = (0.1)(0.01)⁵/((0.05)(0.1)⁵(1)⁸) = 2×10⁻⁸
E = 0.74 – (0.0592/5) log(2×10⁻⁸)
E = 0.74 – 0.01184×( -7.699) ≈ 0.74 + 0.091 ≈ 0.831 V
Nernst Equation Summary
| Variable | Meaning | Units | Typical Values |
|---|---|---|---|
| E | Cell potential | Volts (V) | Depends on system (0.1V to 3V) |
| E° | Standard cell potential | Volts (V) | From standard reduction tables |
| R | Gas constant | J/mol·K | 8.314 |
| T | Temperature | Kelvin (K) | Usually 298K (25°C) |
| n | Electrons transferred | Dimensionless | 1, 2, 3… (from redox equation) |
| F | Faraday’s constant | C/mol | 96485 |
| Q | Reaction quotient | Dimensionless | Productscoefficients/Reactantscoefficients |