Soaps and Detergents
Understanding the Chemistry Behind Cleaning Agents
Soaps and detergents are cleaning agents that play a crucial role in our daily lives. Both have similar properties and purposes but differ significantly in their chemical composition and environmental impact. In this comprehensive guide, we’ll explore the chemistry, properties, and applications of soaps and detergents from an examination perspective.
Key Learning Objectives: By the end of this article, you should understand the chemical composition of soaps and detergents, their preparation methods, cleansing action mechanism, differences between them, and their environmental implications.
Chemistry of Soaps
Definition and Structure
Soaps are sodium or potassium salts of long-chain fatty acids. A typical soap molecule has two distinct regions:
- Hydrophilic head (polar, ionic part): The carboxylate end (-COO–Na+) that is attracted to water
- Hydrophobic tail (non-polar part): The long hydrocarbon chain (typically C15-C17) that is attracted to oil and grease
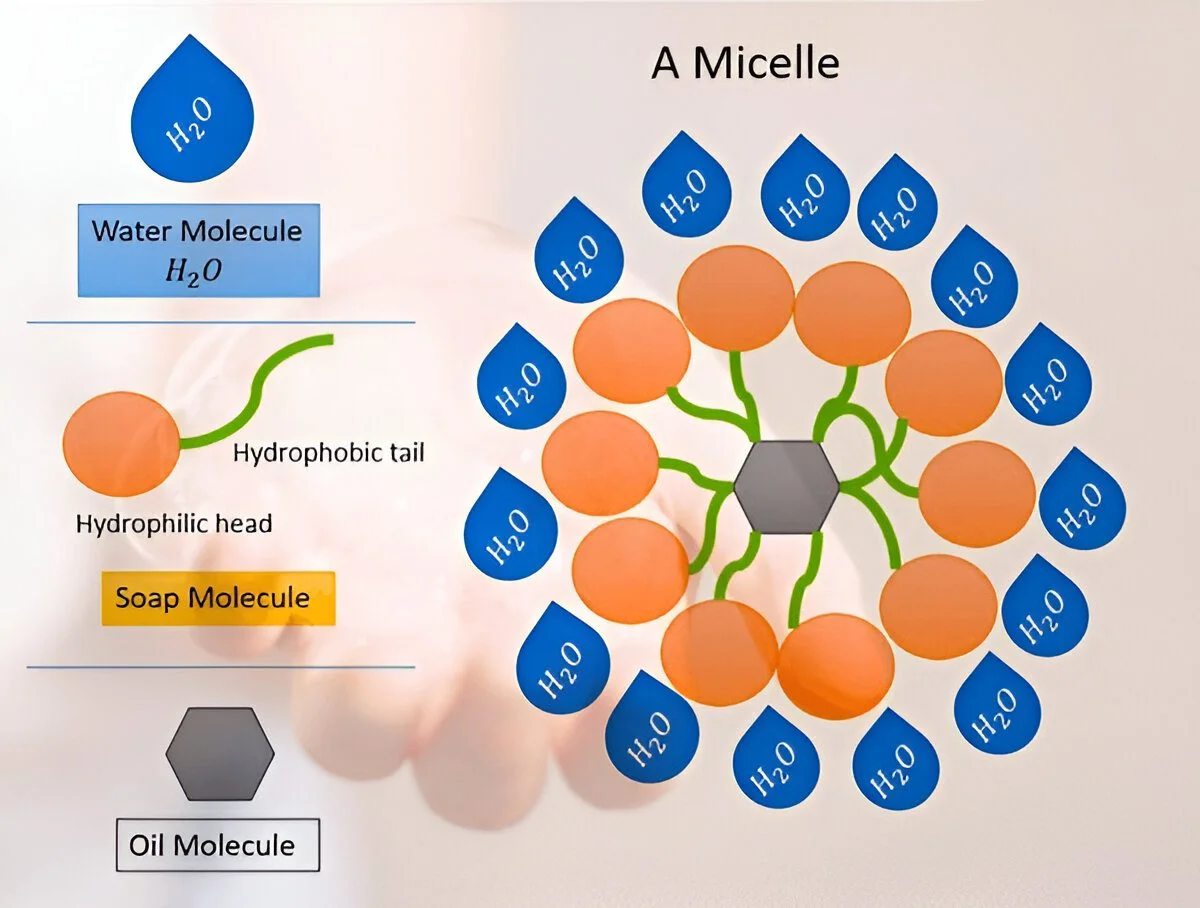
Fig. 1: Structure of a typical soap molecule showing hydrophilic head and hydrophobic tail
Preparation of Soaps
Soaps are prepared by the saponification process, which involves the hydrolysis of fats or oils (triglycerides) with a strong base like sodium hydroxide (NaOH) or potassium hydroxide (KOH).
Fat/Oil (Triglyceride) + 3NaOH → 3 Sodium Soap + Glycerol
(C17H35COO)3C3H5 + 3NaOH → 3C17H35COONa + C3H5(OH)3
Exam Tip: Remember that saponification is a type of hydrolysis reaction that occurs under basic conditions, and it’s irreversible. The chemical formula for soap varies based on the fatty acid used, but a common example is sodium stearate (C17H35COONa).
Types of Soaps
Soaps can be classified based on the alkali used and their specific applications:
| Type | Composition | Properties | Uses |
|---|---|---|---|
| Hard Soap | Sodium salt of fatty acids | Forms less lather, harder texture | Washing clothes, general cleaning |
| Soft Soap | Potassium salt of fatty acids | Forms more lather, softer texture | Shaving creams, liquid hand soaps |
| Medicated Soap | Contains antiseptics or medicinal ingredients | Antibacterial properties | Treating skin infections, antiseptic purposes |
| Transparent Soap | Contains glycerol, sugar, alcohol | Clear appearance | Luxury bathing soaps |
Chemistry of Detergents
Definition and Structure
Detergents are synthetic cleaning agents derived from petrochemicals. Like soaps, they have both hydrophilic and hydrophobic parts, but their chemical structure differs significantly:
- Hydrophilic head: Usually a sulfonate (-SO3–Na+), sulfate (-OSO3–Na+), or sometimes a quaternary ammonium group
- Hydrophobic tail: A long hydrocarbon chain, often branched or with benzene rings
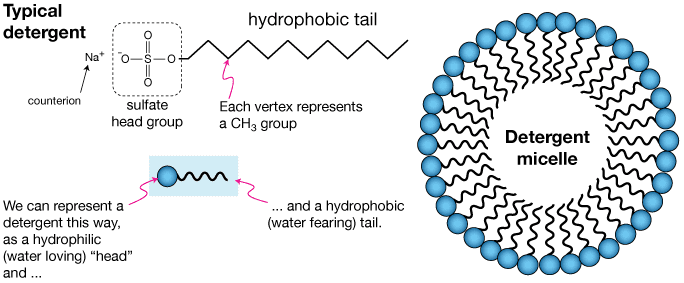
Fig. 2: Structure of a typical detergent molecule with sulfonate head group
Preparation of Detergents
Detergents are typically prepared through the sulfonation or sulfation of hydrocarbons derived from petroleum processing, followed by neutralization with a base.
For alkylbenzene sulfonate detergents:
R-C6H5 + H2SO4 → R-C6H4-SO3H
R-C6H4-SO3H + NaOH → R-C6H4-SO3Na + H2O
Types of Detergents
Detergents can be classified based on their ionic character:
| Type | Chemical Nature | Examples | Uses |
|---|---|---|---|
| Anionic Detergents | Negatively charged hydrophilic head | Sodium lauryl sulfate, Alkylbenzene sulfonates | Laundry detergents, dishwashing liquids |
| Cationic Detergents | Positively charged hydrophilic head | Quaternary ammonium salts | Fabric softeners, antiseptics, hair conditioners |
| Non-ionic Detergents | No charge on hydrophilic head | Polyethylene glycol esters | Low-temperature washing, cleaning delicate fabrics |
| Amphoteric Detergents | Can be cationic or anionic depending on pH | Amino acid-based detergents | Shampoos, personal care products |
Exam Tip: The most commonly used synthetic detergents are linear alkylbenzene sulfonates (LAS). You should be able to identify the different types of detergents based on their ionic character and know their specific applications.
Cleansing Action of Soaps and Detergents
The cleaning action of both soaps and detergents is based on their amphipathic structure (having both hydrophilic and hydrophobic regions). This unique structure allows them to interface between water and oily dirt particles.
Mechanism of Cleansing Action
- Adsorption: When soap/detergent is added to water containing oil or grease, the hydrophobic tails attach to the oil droplets while the hydrophilic heads remain in water.
- Micelle Formation: Multiple soap/detergent molecules surround the oil droplets, forming structures called micelles, where the hydrophobic tails point inward (toward the oil) and the hydrophilic heads point outward (toward the water).
- Emulsification: The oil droplets become suspended in water as an emulsion due to the negative charges on the micelles that repel each other, preventing the oil droplets from coalescing.
- Removal: The emulsified oil particles can be easily washed away with water.
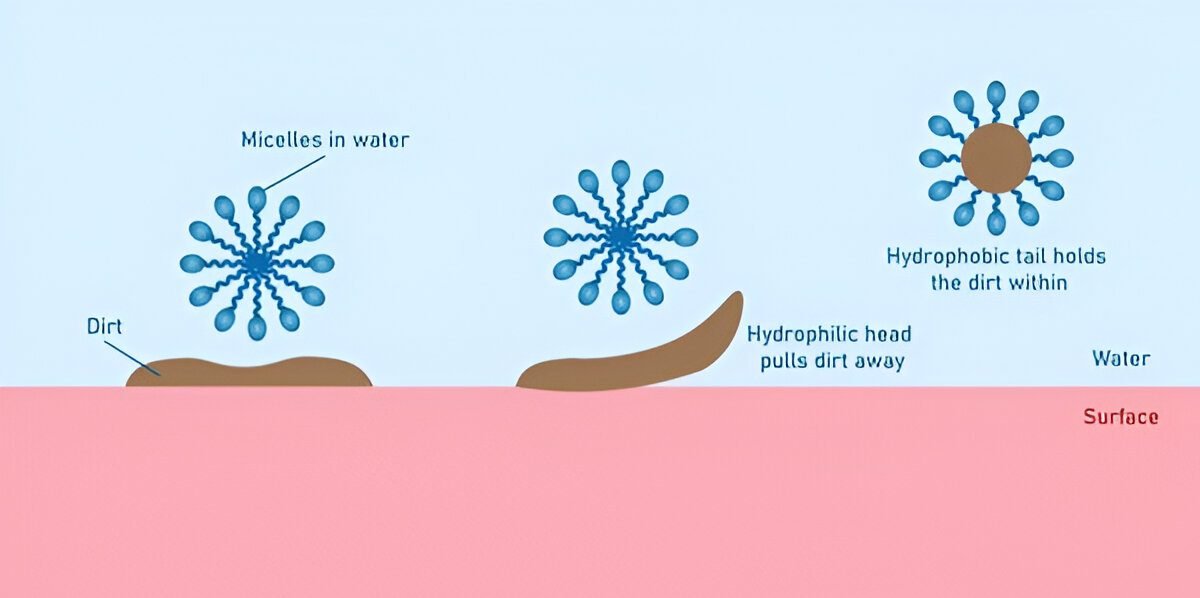
Fig. 3: Micelle formation showing how soap/detergent molecules arrange themselves around oil droplets
Differences Between Soaps and Detergents
| Property | Soaps | Detergents |
|---|---|---|
| Chemical Composition | Sodium or potassium salts of fatty acids | Sodium salts of sulfonated hydrocarbons |
| Origin | Natural (animal fats or vegetable oils) | Synthetic (petroleum products) |
| Effectiveness in Hard Water | Poor (forms insoluble calcium and magnesium salts) | Good (doesn’t form precipitates with hard water ions) |
| Effectiveness in Acidic Medium | Poor (forms insoluble fatty acids) | Good (remains active in acidic medium) |
| Biodegradability | Highly biodegradable | Varies (linear chains are biodegradable, branched are not) |
| pH of Solution | Basic (pH 9-10) | Neutral to slightly alkaline |
| Cost | Generally less expensive | Generally more expensive |
Hard Water Problem
One of the key differences between soaps and detergents is their behavior in hard water. Hard water contains calcium (Ca2+) and magnesium (Mg2+) ions that react with soap molecules to form insoluble precipitates called “soap scum”.
2C17H35COONa + Ca2+ → (C17H35COO)2Ca↓ + 2Na+
(Sodium stearate + Calcium ion → Calcium stearate (insoluble) + Sodium ion)
This precipitation reduces the effectiveness of soap and leaves a residue on fabric and surfaces. Detergents, however, form soluble salts with Ca2+ and Mg2+ ions, making them effective even in hard water.
Exam Tip: You may be asked to write the balanced chemical equation for the reaction between soap and hard water ions, or to explain why detergents work better in hard water. Remember that the key difference is the solubility of the calcium and magnesium salts formed.
Environmental Impact
Soaps
Soaps are generally considered more environmentally friendly because:
- They are produced from renewable resources (plant oils or animal fats)
- They are readily biodegradable by microorganisms
- They don’t persist in the environment for long periods
Detergents
Environmental concerns with detergents include:
- Traditional branched-chain alkylbenzene sulfonates (ABS) are non-biodegradable
- Phosphate additives in detergents contribute to eutrophication (excessive algal growth in water bodies)
- Modern linear alkylbenzene sulfonates (LAS) are biodegradable, addressing some environmental concerns
Note: Many countries have banned or restricted phosphates in detergents due to their environmental impact. Modern detergent formulations often use alternative builders like zeolites or polycarboxylates.
Applications and Importance
Common Applications
- Personal Hygiene: Bath soaps, shampoos, hand washes
- Laundry: Washing powders, liquid detergents, fabric softeners
- Dishwashing: Dishwashing liquids, automatic dishwasher detergents
- Industrial Cleaning: Heavy-duty degreasers, floor cleaners
- Specialty Applications: Medical disinfectants, car wash products
Components of Modern Detergent Formulations
Commercial detergents contain several additives beyond the basic surfactant:
- Builders: Enhance cleaning efficiency by softening water (e.g., sodium tripolyphosphate, zeolites)
- Enzymes: Break down specific stains (e.g., proteases for protein stains, lipases for fat stains)
- Bleaching Agents: Remove colorful stains (e.g., sodium perborate)
- Optical Brighteners: Make clothes appear whiter by converting UV light to visible blue light
- Fragrances: Provide pleasant smell
- Foam Stabilizers: Maintain foam during washing
Exam Focus Summary
Key Concepts to Remember
- Structure and Properties: Both soaps and detergents have amphipathic structures with hydrophilic and hydrophobic parts, enabling them to interact with both water and oil.
- Chemical Composition: Soaps are sodium/potassium salts of fatty acids; detergents are typically sulfonated or sulfated derivatives of hydrocarbons.
- Preparation: Soaps are prepared by saponification of fats/oils; detergents by sulfonation/sulfation of petroleum-derived hydrocarbons.
- Cleansing Action: Both work through micelle formation and emulsification of oily dirt particles.
- Hard Water Performance: Soaps form insoluble precipitates in hard water; detergents remain effective.
- Environmental Impact: Soaps are more biodegradable; some detergents can cause environmental problems.
Exam Strategy: For numerical problems involving soap preparation, remember to use the correct molecular weights and stoichiometry. For conceptual questions, focus on the structure-property relationships and the specific advantages of each type of cleaning agent.
Practice Questions
Question 1: Write the balanced chemical equation for the saponification of glyceryl tristearate (a fat) with sodium hydroxide.
Question 2: Explain why detergents work better than soaps in hard water. Include relevant chemical equations in your answer.
Question 3: Describe the mechanism of cleansing action of soaps, explaining each step with reference to the hydrophilic and hydrophobic parts of soap molecules.
Question 4: Compare and contrast anionic, cationic, and non-ionic detergents with respect to their chemical structures and applications.
Question 5: A student claims that all detergents are environmentally harmful while all soaps are environmentally friendly. Evaluate this statement, providing scientific evidence to support your answer.



