The initial element of the periodic table is hydrogen. Opening Statement Hydrogen, represented by the symbol H, occupies the initial position in the periodic table and has an atomic number of 1. It is the lightest and most basic element that has been identified, and it accounts for approximately 75% of the normal matter in the universe. Hydrogen is a critical element in industrial applications, scientific research, and daily existence due to its distinctive properties.
Exploration and History
Henry Cavendish, an English physicist, was the first to identify hydrogen as a distinct element in 1766. He dubbed it “inflammable air” because of its ability to form water upon combustion. Antoine Lavoisier, a French chemist, later coined the term “hydrogen,” which is derived from Greek words that mean “water-former.”
Physical Properties of Hydrogen
Hydrogen possesses remarkable physical characteristics:
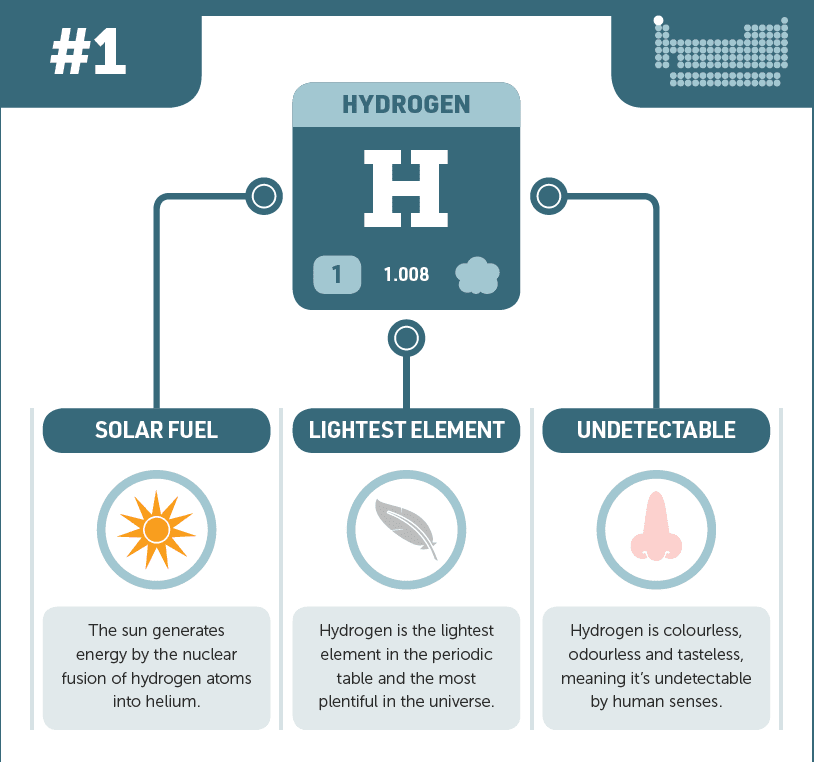
Under typical circumstances, it is a colorless, odorless, and tasteless vapor.
Molecular Form: Diatomic molecules (H₂) are present.
Density: Hydrogen is the lightest element, with a density 1/14th that of air.
Boiling Point: -252.87°C
Melting Point: -259.14°C
Chemical Properties of Hydrogen
Hydrogen is intensely reactive and forms compounds with most elements. Key chemical properties include:
Combining with oxygen to produce water (H₂O).
Reacting with nitrogen to form ammonia (NH₃), essential in fertilizers.
Acting as a reducing agent in industrial chemical reactions.
Occurrence
Hydrogen is the most prevalent element in the universe. On Earth, it is predominantly found in compounds such as water, hydrocarbons, and organic materials. In stars, hydrogen functions as a fuel for nuclear fusion, producing helium and releasing enormous energy.
Isotopes of Hydrogen
Hydrogen has three naturally occurring isotopes:
Protium (H): The most common isotope, with one proton and no neutrons.
Deuterium (D): Contains one proton and one neutron, used in heavy water for nuclear reactors.
Tritium (T): Contains one proton and two neutrons; it is radioactive and plays a role in nuclear fusion research.
Applications of Hydrogen
Hydrogen’s versatility permits for its use in various fields:
Energy: Hydrogen fuel cells generate electricity with water as the only by-product, making it a pure energy source.
Chemical Industry: A critical component for producing ammonia, methanol, and other industrial compounds.
Food Industry: Hydrogenation of vegetable lipids to produce margarine.
Aerospace: Widely used as rocket fuel owing to its high energy yield.
Metallurgy: Employed as a reducing agent in metal refining processes.
Hydrogen Economy
The hydrogen economy concept envisions hydrogen as a principal energy carrier of the future. It has the potential to replace fossil fuels in transportation, power generation, and industrial sectors, substantially reducing greenhouse gas emissions.
Challenges and Future Potential
Despite its potential, hydrogen adoption confronts some challenges:
Production Costs: Extracting hydrogen from water or hydrocarbons requires significant energy and expense.
Storage and Transport: Low density and high flammability make hydrogen difficult to store and transport securely.
Infrastructure: Developing a robust system for hydrogen use requires substantial investment.
Nevertheless, with advancements in technology and increased global focus on sustainability, hydrogen is poised to play a critical role in our transition to greener energy systems.
Conclusion
Hydrogen, the simplest element in the periodic table, is vital to both the universe and human progress. Its wide-ranging applications in energy, industry, and research underline its importance. As a clean and renewable energy carrier, hydrogen contains immense potential to shape a sustainable and eco-friendly future.

